solo para uso en investigación
Necrostatin-1 (Nec-1) Inhibidor de RIPK1
Cat. No.S8037
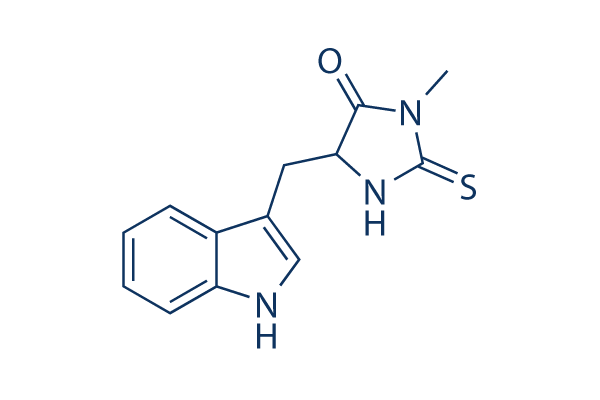
Estructura química
Peso molecular: 259.33
Control de calidad
| Dianas relacionadas | Bcl-2 Caspase PD-1/PD-L1 Ferroptosis p53 Apoptosis related Synthetic Lethality STAT TNF-alpha Ras |
|---|---|
| Otros RIP kinase Inhibidores | Necrostatin 2 racemate (Nec-1s) GSK872 Mito-TEMPO GSK'963 RIPA-56 GSK2982772 Resibufogenin GSK583 HS-1371 GSK2983559 (compound 3) |
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| HT-22 | Cytotoxicity Assay | 10 μM | 12 h | protects against cell death induced by 5 mmol/L glutamate | 17760869 | |
| Jurkat | Function Assay | 200 μm | 30 min | reduces Naegleria fowleri-induced reactive oxygen species (ROS) generation | 21535020 | |
| Jurkat | Cytotoxicity Assay | 50/ 100/200 μm | 1/3 h | reduces Naegleria fowleri-induced cytotoxicity | 21535020 | |
| SW13 | Cell Viability Assay | 100 μM | 24 h | DMSO | increases cellular survival | 22136818 |
| 8505c | Cell Viability Assay | 100 μM | 24 h | DMSO | increases cellular survival | 22136818 |
| TPC-1 | Cell Viability Assay | 100 μM | 24 h | DMSO | increases cellular survival | 22136818 |
| L929sA | Apoptosis Assay | 10 μM | 1 h | abrogates the interaction of caspase-8 with FADD | 22362767 | |
| L929sA | Apoptosis Assay | 10 μM | 1 h | rescues cells expressing RIPK1ΔID from TNF-induced apoptosis | 22362767 | |
| L929sA | Apoptosis Assay | 10 μM | 1 h | inhibits the apoptotic response to TNF | 22362767 | |
| K562/Adr | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| K562 | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| HL60/Adr | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| HL60 | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| K562/Adr | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| K562 | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| HL60/Adr | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| HL60 | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| K562/Adr | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| K562 | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| HL60/Adr | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| HL60 | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| SH-EP | Apoptosis Assay | 10 μM | 72 h | inhibits IAP inhibitor- and Lexatumumab-induced apoptosis | 22890322 | |
| NIH3T3 | Function Assay | 10/50 μM | 1/3 h | ameliorates TNFα-driven complex formation | 23261677 | |
| HT-22 | Function Assay | 25 μM | 0–30 min | DMSO | inhibits ERK Activation induced by glutamate | 23307752 |
| HT-22 | Cell Viability Assay | 10 μM | 12 h | DMSO | protects against glutamate-induced cell death | 23307752 |
| KMS-12-BM | Cytotoxicity Assay | 90 µM | 1 h | blocks BAY 11-7082 induced rapid cell swelling | 23527154 | |
| MM.1S | Cytotoxicity Assay | 90 µM | 1 h | blocks BAY 11-7082 induced rapid cell swelling | 23527154 | |
| ΔN-Karpas 299 | Cytotoxicity Assay | 20 μM | 16 h | inhibits CD30-induced cell death | 23545938 | |
| MEFs | Function Assay | 20 μM | 1/2/4 h | suppresses TNFα-induced RIPK1 phosphorylation | 23727581 | |
| MEFs | Cytotoxicity Assay | 2/6/20 μM | 18 h | inhibits TNFα-induced cell death in RelA KO MEFs | 23727581 | |
| RMS13 | Cell Viability Assay | 40 μg/ml | 24 h | rescues GX15-070-induced loss of cell viability | 23744296 | |
| TE671 | Cell Viability Assay | 40 μg/ml | 24 h | rescues GX15-070-induced loss of cell viability | 23744296 | |
| U87 | Function Assay | 1 mmol/L | 1.5-3 h | suppresses the expression of RIP-1 caused by shikonin | 23840441 | |
| C6 | Function Assay | 1 mmol/L | 1.5-3 h | suppresses the expression of RIP-1 caused by shikonin | 23840441 | |
| U87 | Cytotoxicity Assay | 1 mmol/L | 3 h | blocks shikonin induced necrosis | 23840441 | |
| C6 | Cytotoxicity Assay | 1 mmol/L | 3 h | blocks shikonin induced necrosis | 23840441 | |
| U87 | Cell Viability Assay | 1 mmol/L | 3 h | attenuates Shikonin induced glioma cell death | 23840441 | |
| C6 | Cell Viability Assay | 1 mmol/L | 3 h | attenuates Shikonin induced glioma cell death | 23840441 | |
| L929 | Function Assay | 5 μg/ml | 24 h | blocks zVAD induced necroptosis and autophagy | 23941769 | |
| L929 | Function Assay | 2 μg/ml | 24 h | promots caspase-6 (p20) activity and procaspase-6 cleavage | 23941769 | |
| L929 | Growth Inhibition Assay | 2/5 μg/ml | 24 h | reverses the cell growth inhibition and cell death induced by TNFα alone as well as TNFα + zVAD | 23941769 | |
| L929 | Function Assay | 2/5 μg/ml | 24 h | reversed the autophagy induced by TNFα alone as well as TNFα + zVAD | 23941769 | |
| NRK-52E | Cell Viability Assay | 20 μM | 24 h | inhibits increased Drp1 protein expression after TNF-α Stimulation and ATP Depletion | 24351845 | |
| NRK-52E | Cell Viability Assay | 20 μM | 24 h | increases cell viability after TNF-α Stimulation and ATP Depletion | 24351845 | |
| NRK-52E | Cell Viability Assay | 20 μM | 24 h | protects cells from cell death caused by ischemia injury | 24351845 | |
| AGS | Cell Viability Assay | 60 μm | 1 h | prevents shikonin-induced cell death | 24463199 | |
| L-540 | Cell Viability Assay | 60 μm | 1 h | reduces the Givinostat/Sorafenib-induced cell death | 24561519 | |
| L-540 | Function Assay | 60 μm | 1 h | prevents the mitochondrial membrane depolarization | 24561519 | |
| L-540 | Function Assay | 60 μm | 1 h | prevents the generation of ROS | 24561519 | |
| SK-Hep1 | Function Assay | 60 μM | 18 h | blocks β-lapachone-mediated PAR accumulation and AIF translocation to the cytosol | 24832602 | |
| SK-Hep1 | Function Assay | 60 μM | 18 h | inhibits β-Lapachone-induced leakage of HMGB-1 | 24832602 | |
| SK-Hep1 | Function Assay | 60 μM | 18 h | blocks β-lapachone-induced morphological change, cell death and PI uptake | 24832602 | |
| OHC | Function Assay | 300 μM | DMSO | increases the number of apoptotic OHCs without altering the levels of CC8 after noise exposure | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | diminishes noise-induced AMPK activation | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | results in a reduction of noise-induced RIP1 and RIP3 immunofluorescence | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | decreases noise-induced swollen nuclei | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | increases noise-induced condensed nuclei | 24874734 | |
| Huh7 | Cell Viability Assay | 50 µM | 24/48 h | DMSO | prevents cell death of rAdHCV co-infected Huh7 cells | 24973240 |
| L929 | Cell Viability Assay | 30 μM | 1 h | inhibits TNF-α-induced cleavage of Topo I | 25095742 | |
| L929 | Cell Viability Assay | 30 μM | 1 h | inhibits TNF-α-induced loss of cell viability | 25095742 | |
| L929-A | Function Assay | 50 μM | 12 h | inhibits the TNFα-induced loss of mitochondrial membrane permeability | 25398540 | |
| L929 | Function Assay | 50 μM | 12 h | inhibits TNFα-induced Bid cleavage | 25398540 | |
| L929-N | Function Assay | 50 μM | 12 h | blocks the cleavage of Caspase-3 and PARP | 25398540 | |
| L929-A | Function Assay | 50 μM | 12 h | blocks the cleavage of Caspase-3 and PARP | 25398540 | |
| L929-N | Cell Viability Assay | 50 μM | 24 h | blocks TNFα-induced cell death | 25398540 | |
| L929-A | Cell Viability Assay | 50 μM | 24 h | blocks TNFα-induced cell death | 25398540 | |
| KMS-12-PE | Cell Viability Assay | 60 μM | 5 h | inhibits SHK-induced cell death | 25530098 | |
| SGC-7901 | Cell Viability Assay | 30 μM | 1 h | suppresses oxaliplatin-mediated cell death | 25767076 | |
| BxPC-3 | Function Assay | 20 μM | 24 h | decreases the early necrotic cells | 26000607 | |
| MiaPaCa-2 | Function Assay | 20 μM | 24 h | decreases the early necrotic cells | 26000607 | |
| NCI-H28 | Cell Viability Assay | 10 μM | 24 h | prevents DAPE-induced reduction of NCI-H28 cell viability | 26004138 | |
| BMDM | Function Assay | 10 μM | 30 min | protects cells from TAKI-induced LDH release | 26381601 | |
| MEFs | Cell Viability Assay | 10 μM | 48 h | DMSO | inhibits zVAD-promoted death of CNOT3-depleted MEFs | 26437789 |
| A549 | Cell Viability Assay | 50 μM | 24 h | inhibits MMS-induced cell death | 26472723 | |
| Jurkat T | Necroptosis assay | Inhibition of TNF-alpha-induced necroptosis in FADD-deficient human Jurkat T cells, EC50 = 0.05 μM. | 18467094 | |||
| Jurkat | Function assay | Inhibition of endogenous RIP1 autophosphorylation in human Jurkat cells, EC50 = 0.182 μM. | 18408713 | |||
| Sf9 | Function assay | 30 mins | Inhibition of recombinant human GST-fused RIPK1 (1 to 497 residues) expressed in baculovirus infected insect Sf9 cells in presence of 32P-gamma-ATP after 30 mins by autoradiogram-based Western blot method, IC50 = 0.182 μM. | 28280261 | ||
| Jurkat T | Necroptosis assay | Effective concentration required for inhibition of necroptosis in FADD deficient Jurkat T cells treated with TNF-alpha, EC50 = 0.49 μM. | 16153840 | |||
| Jurkat | Necroptosis assay | Inhibition of cellular necroptosis in TNFalpha treated FADD deficient human Jurkat cells, EC50 = 0.49 μM. | 18408713 | |||
| Jurkat T | Necroptosis assay | 30 uM | 24 hrs | Inhibition of necroptosis in TNF-alpha-induced human Jurkat T cells assessed as cell viability at 30 uM after 24 hrs | 18467094 | |
| L929 | Necroptosis assay | 30 uM | 24 hrs | Inhibition of necroptosis in zVAD-induced mouse L929 cells assessed as cell viability at 30 uM after 24 hrs | 18467094 | |
| L929 | Necroptosis assay | 30 uM | 24 hrs | Inhibition of necroptosis in TNF-alpha-induced mouse L929 cells assessed as cell viability at 30 uM after 24 hrs | 18467094 | |
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necrotic cell death in mouse 3T3 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of TNFalpha and zVAD.fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necrotic cell death in mouse 3T3 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of FasL and zVAD.fmk | 16408008 | ||
| MEF | Cell death assay | 16 hrs | Inhibition of death receptor signaling mediated necrotic cell death in SV40 transformed mouse MEF cells assessed as cell viability after 16 hrs by ATP based viability assay in presence of TNFalpha, CHX and zVAD.fmk | 16408008 | ||
| IEC18 | Cell death assay | Inhibition of death receptor signaling mediated necrotic cell death in rat IEC18 cells assessed as cell viability in presence of TNFalpha and zVAD.fmk | 16408008 | |||
| HL60 | Cell death assay | Inhibition of death receptor signaling mediated necrotic cell death in human HL60 cells assessed as cell viability in presence of TNFalpha and zVAD.fmk | 16408008 | |||
| L929 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necrotic cell death in mouse L929 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of TNFalpha | 16408008 | ||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as nuclear condensation by bright field microscopy in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as organelle swelling by bright field microscopy in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as early loss of plasma membrane integrity by bright field microscopy in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as appearance of translucent cytosol in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of nuclear condensation by bright field microscopy in presence of TNFalpha | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of organelle swelling by bright field microscopy in presence of TNFalpha | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of early loss of plasma membrane integrity by bright field microscopy in presence of TNFalpha | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of appearance of translucent cytosol in presence of TNFalpha | 16408008 | |||
| U937 | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human U937 cells assessed as cell viability after 48 hrs by ATP based viability assay in presence of TNFalpha and zVAD-fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of TNFalpha and zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD assessed as decreased levels of PE-conjugated LC3-II (autophagy marker) after 24 hrs by Western blot method in presence of TNFalpha | 16408008 | ||
| L929 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse L929 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of TNFalpha | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of TNFalpha and zVAD-fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of FasL and zVAD-fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of rapamycin | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing FKBP12-based dimerization domain assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP K45M mutant assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase domain assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing FKBP12-based dimerization domain assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP K45M mutant assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase domain assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Sf9 | Function assay | Inhibition of human RIP1 K45M mutant autophosphorylation expressed in Sf9 cells | 18408713 | |||
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against FasL-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by FasL stimulation measured after 20 hrs by Alamar blue assay | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against CHX-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by CHX stimulation by Alamar blue assay | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against Z-VAD-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by Z-VAD stimulation by Alamar blue assay | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against FasL-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by FasL stimulation measured after 20 hrs by phase contrast microscopy | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against CHX-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by CHX stimulation by phase contrast microscopy | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against Z-VAD-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by Z-VAD stimulation by phase contrast microscopy | 29541357 | |
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 259.33 | Fórmula | C13H13N3OS |
Almacenamiento (Desde la fecha de recepción) | |
|---|---|---|---|---|---|
| Nº CAS | 4311-88-0 | Descargar SDF | Almacenamiento de soluciones madre |
|
|
| Sinónimos | Nec-1 | Smiles | CN1C(=O)C(NC1=S)CC2=CNC3=CC=CC=C32 | ||
Solubilidad
|
In vitro |
DMSO
: 57 mg/mL
(219.79 mM)
Water : Insoluble Ethanol : Insoluble |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| Características |
A powerful tool for characterizing the role of necroptosis with characterized primary target.
|
|---|---|
| Targets/IC50/Ki |
IDO
RIP1
(293T cells) 490 nM(EC50)
|
| In vitro |
Necrostatin-1 (1-100 μM) inhibe la autofosforilación de RIP1 sobreexpresada y endógena. Se ha encontrado que RIP1 es el objetivo celular primario responsable de la actividad antinecroptosis de este compuesto. Este químico suprime eficientemente la muerte celular necroptótica desencadenada por una variedad de estímulos en una variedad de tipos celulares. Previamente identificado como un inhibidor de moléculas pequeñas de la necroptosis, inhibe la necroptosis inducida por RIP kinase e inhibe la necroptosis inducida por TNF-α en células Jurkat con una EC50 de 490 nM. |
| Ensayo de quinasa |
Ensayo de quinasa RIP1
|
|
La fosforilación de RIP1 requiere su actividad quinasa. Se transfectan construcciones de expresión de RIP1 de tipo salvaje (WT) marcado con FLAG o un mutante puntual inactivo de quinasa de RIP1 (K45M) en células 293T y se realiza el ensayo de quinasa RIP1 como se describe en los Métodos en presencia de [γ-32P]ATP durante 30 min a 30℃. Las muestras se someten a SDS-PAGE y la banda RIP1 se visualiza mediante autorradiografía. Las intensidades relativas de las bandas radiactivas se cuantifican y se muestran (proporción) en este y todos los demás autorradiogramas. Paralelamente a las reacciones de quinasa, una muestra de perlas se somete a análisis de Western blot utilizando un anticuerpo anti-RIP1 para asegurar cantidades iguales de proteínas en las reacciones de quinasa.
|
|
| In vivo |
Necrostatin-1 (Nec-1) es un inhibidor de molécula pequeña específico de la quinasa 1 (RIPK1) que interactúa con el receptor y que inhibe específicamente la fosforilación de este compuesto. |
Referencias |
|
Aplicaciones
| Métodos | Biomarcadores | Imágenes | PMID |
|---|---|---|---|
| Immunofluorescence | RIP1 / RIP3 |
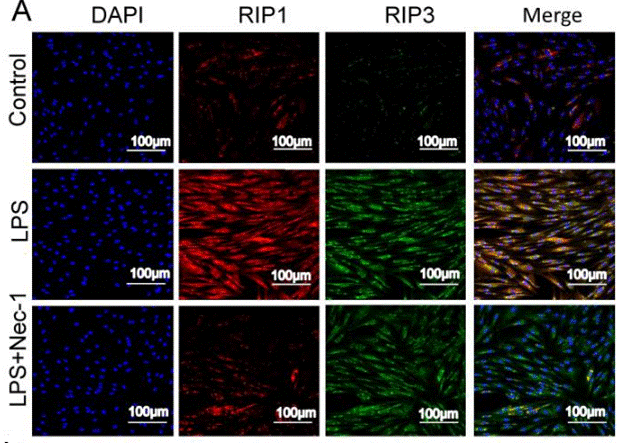
|
30462730 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
