solo para uso en investigación
Aspirin COX inhibidor
Cat. No.S3017
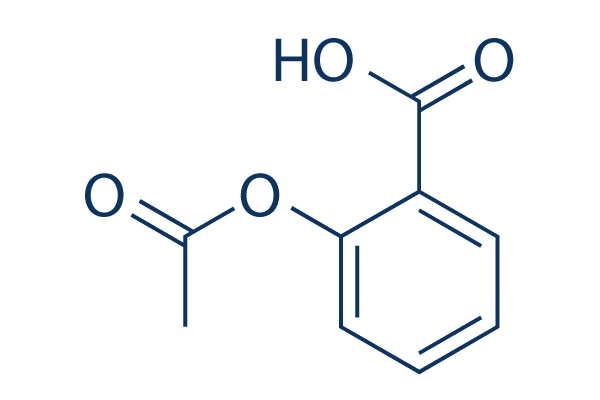
Estructura química
Peso molecular: 180.16
Control de calidad
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| AGS | Function assay | 15 to 60 umol/L after | 12 hrs | Inhibition of Escherichia coli-stimulated IL-8 production in human AGS cells at 15 to 60 umol/L after 12 hrs by ELISA | 20153183 | |
| microglia cells | Antineuroinflammatory assay | 15 mins | Antineuroinflammatory activity in LPS-stimulated rat microglia cells assessed as inhibition of PMA-stimulated TXB2 release preincubated for 15 mins measured 70 mins after PMA challenge, IC50=3.12μM | 22153874 | ||
| HCT116 | Function assay | 1 mM | 6 hrs | Inhibition of TNF-alpha-induced NF-kappaB activation in human HCT116 cells at 1 mM after 6 hrs by luciferase reporter gene assay | 22154834 | |
| SKBR3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human SKBR3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PANC1 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PANC1 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PC3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PC3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| MDA-MB-231 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human MDA-MB-231 cells assessed as inhibition of arachidonic acid-induced PGE2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| THP1 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human THP1 cells assessed as inhibition of arachidonic acid-induced TXB2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as reduction in cell viability after 48 hrs MTT assay | 26750401 | ||
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by E | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in catalase activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spec | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by EL | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced cytotoxicity in rat H9c2 cells assessed as increase in cell viability at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by MTT assay | 27575471 | |
| RAW264.7 | Antiinflammatory assay | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production by measuring nitrite accumulation by Griess method, IC50=43.2μM | 28561586 | |||
| stem cells | Function assay | 100 uM | 5 days | Induction of adipogenesis in human bone marrow-derived mesenchymal stem cells assessed as increase in adiponectin production at 100 uM measured on day 5 in presence of IDX by ELISA | 29398443 | |
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| peritoneal cells | Function assay | 5 hrs | Inhibition of LPS-induced PGE2 production in C57BL6 mouse peritoneal cells measured at 5 hrs time interval by ELISA, IC50=4.08μM | 30006172 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT8 cells after 72 hrs in presence of cinnamaldehyde by MTT assay, IC50=15.6μM | 30037494 | ||
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at G0/G1 phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at S phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as reduction in accumulation at G2/M phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| HaCaT | Antipyretic assay | 100 uM | 2 hrs | Antipyretic activity in human HaCaT cells assessed as inhibition of TNFalpha-induced PGE2 production at 100 uM pre-incubated for 2 hrs before TNFalpha stimulation for 24 hrs by ELISA | 31393125 | |
| OVCAR5 | Function assay | 300 uM to 1 mM | 72 hrs | Inhibition of NAPRT in human OVCAR5 cells assessed as potentiation of NAMPT inhibitor FK866-induced cytotoxicity by measuring reduction in cell viability at 300 uM to 1 mM incubated for 72 hrs by SRB assay | ChEMBL | |
| CCRF-CEM | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human CCRF-CEM cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Jurkat | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Jurkat cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further i | ChEMBL | |
| ML2 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ML2 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NOMO | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NOMO cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NB4 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NB4 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NAMALWA | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NAMALWA cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Daudi | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Daudi cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| Raji | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Raji cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| ARH77 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ARH77 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| RPMI8226 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human RPMI8226 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| U266 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human U266 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NAMALWA | Function assay | 35 mg/kg | 45 days | Potentiation of NAMPT inhibitor 10 mg/kg, ip bid FK866-induced antitumor activity in human NAMALWA cells xenografted in SCID mouse assessed as increase in mouse survival at 35 mg/kg, ip bid up to 45 days | ChEMBL | |
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 180.16 | Fórmula | C9H8O4 |
Almacenamiento (Desde la fecha de recepción) | |
|---|---|---|---|---|---|
| Nº CAS | 50-78-2 | Descargar SDF | Almacenamiento de soluciones madre |
|
|
| Sinónimos | NSC 27223, Acetylsalicylic acid, ASA | Smiles | CC(=O)OC1=CC=CC=C1C(=O)O | ||
Solubilidad
|
In vitro |
DMSO
: 36 mg/mL
(199.82 mM)
Ethanol : 36 mg/mL Water : Insoluble |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| Targets/IC50/Ki |
COX2
COX1
|
|---|---|
| In vitro |
Aspirin inhibe la activación de NF-kappa B, lo que previene la degradación del inhibidor de NF-kappa B, I kappa B, y por lo tanto NF-kappa B se retiene en el citosol. Este compuesto también inhibe la transcripción dependiente de NF-kappa B del potenciador Ig kappa y de la repetición terminal larga (LTR) del virus de la inmunodeficiencia humana (VIH) en células T transfectadas. Su acción y la del salicilato están mediadas en parte por su inhibición específica de IKK-beta, previniendo así la activación por NF-kappaB de genes implicados en la patogénesis de la respuesta inflamatoria. Este producto químico es protector contra la neurotoxicidad provocada por el aminoácido excitatorio glutamato en cultivos neuronales primarios de rata y rebanadas de hipocampo. Desencadena la biosíntesis transcelular de una clase de eicosanoides previamente no reconocida durante las co-incubaciones de células endoteliales de vena umbilical humana (HUVEC) y neutrófilos [leucocitos polimorfonucleares (PMN)]. Este agente evoca una clase única de eicosanoides formados por interacciones de PGHS-2 acetilada y 5-lipoxigenasa. Su tratamiento inhibe la fosforilación de IRS-1 en Ser307, así como la fosforilación de JNK, c-Jun y la degradación de IkappaBalpha en células 3T3-L1 y Hep G2 tratadas con factor de necrosis tumoral (TNF)-alpha. Este tratamiento inhibe la fosforilación de Akt y del objetivo de rapamicina en mamíferos (pero no de la quinasa regulada por señales extracelulares o PKCzeta) en respuesta a TNF-alpha. Rescata la captación de glucosa inducida por insulina en adipocitos 3T3-L1 pretratados con TNF-alpha.
|
Referencias |
|
Aplicaciones
| Métodos | Biomarcadores | Imágenes | PMID |
|---|---|---|---|
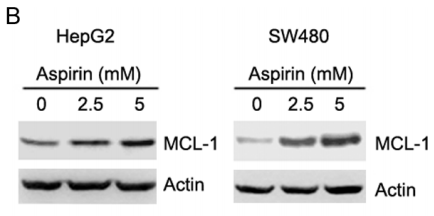
| Western blot | MCL-1 p-AKT / AKT / p-ERK / ERK p-AMPKα / AMPKα / p-ACC / ACC SHH / SMO / GLI1 / Bcl-2 / Foxm1 |
|
26918349 |
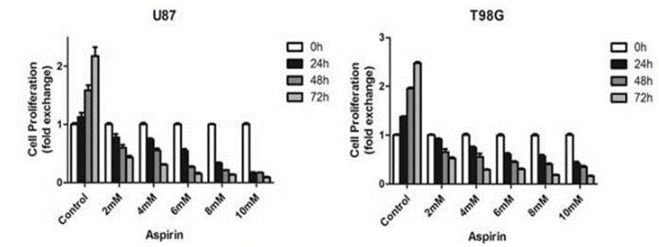
| Growth inhibition assay | Cell proliferation Cell viability |
|
28446712 |
Información del ensayo clínico
(datos de https://clinicaltrials.gov, actualizado el 2024-05-22)
| Número NCT | Reclutamiento | Condiciones | Patrocinador/Colaboradores | Fecha de inicio | Fases |
|---|---|---|---|---|---|
| NCT05960864 | Not yet recruiting | Ankylosing Spondylitis (AS) / Radiographic Axial SpA (r-axSpA)|Non-radiographic Axial Spondyloarthritis (Nr-axSpA)|Axial Psoriatic Arthritis (axPsA)|Acute Anterior Uveitis (AAU)|Crohn Disease (CD)|Ulcerative Colitis (UC)|Reactive Arthritis (ReA) |
Southwest Hospital China |
September 2024 | -- |
| NCT05868525 | Recruiting | Blunt Cerebrovascular Injury |
Loma Linda University |
July 2024 | Phase 4 |
| NCT06197009 | Not yet recruiting | Axial Spondyloarthritis |
Sinocelltech Ltd. |
July 1 2024 | Phase 2 |
| NCT06378398 | Not yet recruiting | Colorectal Neoplasia |
University of Michigan Rogel Cancer Center|National Cancer Institute (NCI) |
May 2024 | Early Phase 1 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
