solo para uso en investigación
Tivantinib c-Met inhibidor
Cat. No.S2753
Estructura química
Peso molecular: 369.42
Control de calidad
| Dianas relacionadas | EGFR VEGFR PDGFR FGFR Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Otros c-Met Inhibidores | Tepotinib Dihexa SGX-523 PHA-665752 Foretinib SU11274 BMS-777607 JNJ-38877605 PF-04217903 Savolitinib (AZD6094) |
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| MNK-45 | Kinase assay | ~10 μM | inhibits c-Met phosphorylation and downstream c-Met signaling pathways | |||
| HT29 | Kinase assay | ~10 μM | inhibits c-Met phosphorylation and downstream c-Met signaling pathways | |||
| MDA-MB-231 | Kinase assay | ~10 μM | inhibits c-Met phosphorylation and downstream c-Met signaling pathways | |||
| NCI-H441 | Kinase assay | ~10 μM | inhibits c-Met phosphorylation and downstream c-Met signaling pathways | |||
| SK-MEL-28 | Growth inhibitory assay | 33 μM | IC50>33 μM | |||
| NCI-H661 | Growth inhibitory assay | 33 μM | IC50>33 μM | |||
| NCI-H446 | Growth inhibitory assay | 33 μM | IC50=7 μM | |||
| MDA-MB-231 | Growth inhibitory assay | 33 μM | IC50=0.55 μM | |||
| DLD-1 | Growth inhibitory assay | 33 μM | IC50=0.53 μM | |||
| A549 | Growth inhibitory assay | 33 μM | IC50=0.59 μM | |||
| SK-OV-3 | Growth inhibitory assay | 33 μM | IC50=0.66 μM | |||
| NCI-H460 | Growth inhibitory assay | 33 μM | IC50=0.6 μM | |||
| A375 | Growth inhibitory assay | 33 μM | IC50=0.42 μM | |||
| NCI-H441 | Growth inhibitory assay | 33 μM | IC50=0.3 μM | |||
| HT29 | Growth inhibitory assay | 33 μM | IC50=0.49 μM | |||
| MKN-45 | Growth inhibitory assay | 33 μM | IC50=0.58 μM | |||
| HT29 | Apoptosis assay | ~10 μM | significantly induces apoptosis by 80-90%. | |||
| MKN-45 | Apoptosis assay | ~10 μM | significantly induces apoptosis by 80-90%. | |||
| MDA-MB-231 | Apoptosis assay | ~10 μM | modestly induces apoptosis by 35%. | |||
| MDA-MB-231/TGL | Growth inhibitory assay | ~100 μM | GI50=1.2 μM | |||
| 1833/TGL | Growth inhibitory assay | ~100 μM | GI50=3.7 μM | |||
| EBC1 | Cytotoxic assay | ~10 μM | inhibits the cell growth. | |||
| SNU638 | Cytotoxic assay | ~10 μM | inhibits the cell growth. | |||
| A549 | Cytotoxic assay | ~10 μM | not affect | |||
| H460 | Cytotoxic assay | ~10 μM | not affect | |||
| HCC827 | Cytotoxic assay | ~10 μM | not affect | |||
| A549 | Function assay | 10 μM | disrupts microtubule | |||
| EBC1 | Function assay | 10 μM | disrupts microtubule | |||
| H460 | Function assay | 10 μM | inhibits tubulin polymerization | |||
| K562/VCR | Cytotoxic assay | ~10 μM | shows cytotoxic activity | |||
| CEM/VBL | Cytotoxic assay | ~10 μM | shows cytotoxic activity | |||
| U266 | Cytotoxic assay | ~3 μM | IC50=1.1 μM | |||
| OPM-2 | Cytotoxic assay | ~3 μM | IC50=1.8 μM | |||
| MM.1S | Cytotoxic assay | ~3 μM | IC50=1.6 μM | |||
| MM.1R | Growth inhibitory assay | 3 μM | inhibits cell growth by 49% | |||
| RPMI-8226 | Cytotoxic assay | ~3 μM | IC50=0.9 μM | |||
| ANBL-6 | Cytotoxic assay | 1 μM | induces cell death by more than 50% | |||
| ANLB-6/V10R | Cytotoxic assay | 1 μM | induces cell death by more than 50% | |||
| KAS-6/1 | Cytotoxic assay | 1 μM | induces cell death by more than 50% | |||
| KAS-6/V10R | Cytotoxic assay | 1 μM | induces cell death by more than 50% | |||
| KAS-6/R10R | Cytotoxic assay | 1 μM | induces cell death by more than 50% | |||
| 8226/S | Growth inhibitory assay | 3 μM | inhibits cell growth by 54% | |||
| 8226/LR-5 | Growth inhibitory assay | 3 μM | inhibits cell growth by 54% | |||
| Huh7 | Cytotoxic assay | ~4.8 μM | DMSO | IC50=9.9 nM | ||
| Hep3B | Cytotoxic assay | ~4.8 μM | DMSO | IC50=448.7 nM | ||
| HepG2 | Cytotoxic assay | ~4.8 μM | DMSO | IC50=139.77 nM | ||
| Chang | Cytotoxic assay | ~4.8 μM | DMSO | IC50=448.7 nM | ||
| Huh7 | Function assay | 1.6 μM | DMSO | causes a G2/M cell cycle arrest | ||
| Hep3B | Function assay | 1.6 μM | DMSO | causes a G2/M cell cycle arrest | ||
| HepG2 | Function assay | 1.6 μM | DMSO | causes a G2/M cell cycle arrest | ||
| Chang | Function assay | 1.6 μM | DMSO | causes a G2/M cell cycle arrest | ||
| MHCC97L | Growth inhibitory assay | ~10 μM | DMSO | IC50=315 nM | ||
| MHCC97H | Growth inhibitory assay | ~10 μM | DMSO | IC50=368 nM | ||
| Huh7 | Growth inhibitory assay | ~10 μM | DMSO | IC50=265 nM | ||
| HepG2 | Growth inhibitory assay | ~10 μM | DMSO | IC50=392 nM | ||
| MHCC97L | Function assay | 1 μM | DMSO | induces microtubules depolymerization | ||
| Huh7 | Function assay | 1 μM | DMSO | induces microtubules depolymerization | ||
| MHCC97L | Apoptosis assay | 1 μM | DMSO | induces apoptosis | ||
| Huh7 | Apoptosis assay | 1 μM | DMSO | induces apoptosis | ||
| C3H 10T1/2 mouse fibroblasts | Kinase assay | 25 μM | DMSO | reduces Histone H3 and H4 acetylation levels | ||
| H23 | Growth inhibitory assay | 25 μM | DMSO | significantly inhibits cell growth. | ||
| WM35 | Growth inhibitory assay | 10 μM | DMSO | significantly inhibits cell growth. | ||
| NIH 3T3 | Growth inhibitory assay | 10 μM | DMSO | does not have a significant inhibitory effect | ||
| H838 | Growth inhibitory assay | 10 μM | DMSO | does not have a significant inhibitory effect | ||
| H1395 | Growth inhibitory assay | 10 μM | DMSO | does not have a significant inhibitory effect | ||
| Quiescent S2 | Kinase assay | 30 μM | DMSO | completely abrogates TSA-induced hyperacetylation of H3K4me3 histones | ||
| PC3 | Apoptosis assay | 20 μM | DMSO | induces apoptosis | ||
| Du145 | Apoptosis assay | 20 μM | DMSO | induces apoptosis | ||
| LNCaP | Apoptosis assay | 20 μM | DMSO | induces apoptosis | ||
| LAPC-4 | Apoptosis assay | 20 μM | DMSO | induces apoptosis | ||
| LNCaP | Function assay | 20 μM | DMSO | decreases PSA secretion and p65 expression levels | ||
| LAPC-4 | Function assay | 20 μM | DMSO | decreases PSA secretion and p65 expression levels | ||
| Kasumi-1 | Growth inhibitory assay | ~50 μM | DMSO | inhibits cell proliferation | ||
| SKNO-1 | Growth inhibitory assay | ~50 μM | DMSO | inhibits cell proliferation | ||
| Kasumi-1 | Kinase assay | ~10 μM | DMSO | reduces expression of acetylated histone H3, c-kit and bcl-2 | ||
| SKNO-1 | Kinase assay | ~10 μM | DMSO | reduces expression of acetylated histone H3, c-kit and bcl-2 | ||
| A549 | Function assay | 10 μM | DMSO | enhances mitotic catastrophe | ||
| NRK-52E | Function assay | 10 μM | DMSO | inhibits Ang II-induced STAT3 nuclear translocation and the expression of TGF-β1, collagen IV and fibronectin | ||
| PC12 | Growth inhibitory assay | ~12.5 μM | DMSO | prevents TSA-induced neurite formation | ||
| HPMCs | Function assay | reverses epithelial to mesenchymal transition of human peritoneal mesothelial cells | ||||
| A549 | Function assay | ~50 μM | DMSO | affects the viral life cycle and host response | ||
| RAW264.7 | Function assay | ~30 μM | DMSO | reduces pro-inflammatory gene expression | ||
| MEMM | Kinase assay | 15 µM | DMSO | decreases acetylation of histone H3 | ||
| MEMM | Growth inhibitory assay | ~20 µM | DMSO | inhibits cell proliferation | ||
| MEMM | Apoptosis assay | 15 µM | DMSO | induces the presence of the apoptosis protein, cleaved Caspase-3 | ||
| T47D | Growth inhibitory assay | 10 μM | DMSO | IC50=72 nM | ||
| ZR-75-1 | Growth inhibitory assay | 10 μM | DMSO | IC50=79 nM | ||
| BT474 | Growth inhibitory assay | 10 μM | DMSO | IC50=86 nM | ||
| HCC1954 | Growth inhibitory assay | 10 μM | DMSO | IC50=119 nM | ||
| MDA-MB-453 | Growth inhibitory assay | 10 μM | DMSO | IC50=975 nM | ||
| MDA-MB-468 | Growth inhibitory assay | 10 μM | DMSO | IC50=3208 nM | ||
| SkBr3 | Growth inhibitory assay | 10 μM | DMSO | IC50>10,000 nM | ||
| MDA-MB-231 | Growth inhibitory assay | 10 μM | DMSO | IC50>10,000 nM | ||
| HCT116 | Growth inhibitory assay | 10 μM | DMSO | IC50=5836 nM | ||
| HT29 | Growth inhibitory assay | 10 μM | DMSO | IC50>10,000 nM | ||
| HFF | Growth inhibitory assay | 10 μM | DMSO | IC50=7615 nM | ||
| HN5 | Growth inhibitory assay | 10 μM | DMSO | IC50>10,000 nM | ||
| 786-0 | Growth inhibitory assay | 10 μM | DMSO | IC50=4009 nM | ||
| H157 | Growth inhibitory assay | 10 μM | DMSO | IC50=2642 nM | ||
| NCI-H460 | Growth inhibitory assay | 10 μM | DMSO | IC50>2,500 nM | ||
| SKOV-3 | Growth inhibitory assay | 10 μM | DMSO | IC50=2126 nM | ||
| OVCAR-3 | Growth inhibitory assay | 10 μM | DMSO | IC50=2918 nM | ||
| BXPC3 | Growth inhibitory assay | 10 μM | DMSO | IC50=3141 nM | ||
| MiaPaCa | Growth inhibitory assay | 10 μM | DMSO | IC50=5433 nM | ||
| PANC-1 | Growth inhibitory assay | 10 μM | DMSO | IC50=8681 nM | ||
| LNCaP | Growth inhibitory assay | 10 μM | DMSO | IC50=147 nM | ||
| DU145 | Growth inhibitory assay | 10 μM | DMSO | IC50=3812 nM | ||
| PC3 | Growth inhibitory assay | 10 μM | DMSO | IC50>10,000 nM | ||
| BT474 | Kinase assay | 10 μM | DMSO | inhibits pGSK3β with IC50 of 160 nM | ||
| 786-0 | Kinase assay | 10 μM | DMSO | inhibits pGSK3β with IC50 of 150 nM | ||
| LNCaP | Kinase assay | 10 μM | DMSO | inhibits pGSK3β with IC50 of 43 nM | ||
| PC3 | Kinase assay | 10 μM | DMSO | inhibits pGSK3β with IC50 of 49 nM | ||
| KARPAS-231 | Growth inhibitory assay | 10 μM | DMSO | EC50=41 nM | ||
| CCRFSB | Growth inhibitory assay | 10 μM | DMSO | EC50=155 nM | ||
| SUP B15 | Growth inhibitory assay | 10 μM | DMSO | EC50=197 nM | ||
| SD-1 | Growth inhibitory assay | 10 μM | DMSO | EC50=320 nM | ||
| RS4;11 | Growth inhibitory assay | 10 μM | DMSO | EC50=654 nM | ||
| MN-60 | Growth inhibitory assay | 10 μM | DMSO | EC50=3602 nM | ||
| Tanoue | Growth inhibitory assay | 10 μM | DMSO | EC50=4517 nM | ||
| RCH-ACV | Growth inhibitory assay | 10 μM | DMSO | EC50=152 nM | ||
| SEM | Growth inhibitory assay | 10 μM | DMSO | EC50=202 nM | ||
| KASUMI-2 | Growth inhibitory assay | 10 μM | DMSO | EC50=225 nM | ||
| REH | Growth inhibitory assay | 10 μM | DMSO | EC50=288 nM | ||
| 697 | Growth inhibitory assay | 10 μM | DMSO | EC50=338 nM | ||
| NALM-6 | Growth inhibitory assay | 10 μM | DMSO | EC50=421 nM | ||
| MHH-CALL–3 | Growth inhibitory assay | 10 μM | DMSO | EC50=812 nM | ||
| MHH-CALL–2 | Growth inhibitory assay | 10 μM | DMSO | EC50=2114 nM | ||
| J.GAMMA-1 | Growth inhibitory assay | 10 μM | DMSO | EC50=65 nM | ||
| JR45.01 | Growth inhibitory assay | 10 μM | DMSO | EC50=68 nM | ||
| A3 | Growth inhibitory assay | 10 μM | DMSO | EC50=69 nM | ||
| I 2.1 | Growth inhibitory assay | 10 μM | DMSO | EC50=73 nM | ||
| MOLT-3 | Growth inhibitory assay | 10 μM | DMSO | EC50=74 nM | ||
| P116 | Growth inhibitory assay | 10 μM | DMSO | EC50=78 nM | ||
| J.Cam1.6 | Growth inhibitory assay | 10 μM | DMSO | EC50=79 nM | ||
| I 9.2 | Growth inhibitory assay | 10 μM | DMSO | EC50=80 nM | ||
| LOUCY | Growth inhibitory assay | 10 μM | DMSO | EC50=117 nM | ||
| J.RT3-T3.5 | Growth inhibitory assay | 10 μM | DMSO | EC50=123 nM | ||
| 800000 | Growth inhibitory assay | 10 μM | DMSO | EC50=163 nM | ||
| Jurkat | Growth inhibitory assay | 10 μM | DMSO | EC50=225 nM | ||
| MOLT-4 | Growth inhibitory assay | 10 μM | DMSO | EC50=232 nM | ||
| Molt-16 | Growth inhibitory assay | 10 μM | DMSO | EC50=241 nM | ||
| CEM/C3 | Growth inhibitory assay | 10 μM | DMSO | EC50=257 nM | ||
| CEM/C2 | Growth inhibitory assay | 10 μM | DMSO | EC50=271 nM | ||
| CCRFCEM | Growth inhibitory assay | 10 μM | DMSO | EC50=327 nM | ||
| CEM/C1 | Growth inhibitory assay | 10 μM | DMSO | EC50=382 nM | ||
| SUPTI[VB] | Growth inhibitory assay | 10 μM | DMSO | EC50=619 nM | ||
| CCRF–HSB-2 | Growth inhibitory assay | 10 μM | DMSO | EC50=2117 nM | ||
| I 2.1 | Apoptosis assay | 10 μM | DMSO | induces apoptosis | ||
| I 9.2 | Apoptosis assay | 10 μM | DMSO | induces apoptosis | ||
| A3 | Apoptosis assay | 10 μM | DMSO | induces apoptosis | ||
| RD | Growth inhibitory assay | 10 μM | IC50>10 μM | |||
| Rh41 | Growth inhibitory assay | 10 μM | IC50=33.8 nM | |||
| Rh18 | Growth inhibitory assay | 10 μM | IC50=303 nM | |||
| Rh30 | Growth inhibitory assay | 10 μM | IC50=4.81 μM | |||
| BT-12 | Growth inhibitory assay | 10 μM | IC50>10 μM | |||
| CHLA-266 | Growth inhibitory assay | 10 μM | IC50=1.22 μM | |||
| TC-71 | Growth inhibitory assay | 10 μM | IC50=2.52 μM | |||
| CHLA-9 | Growth inhibitory assay | 10 μM | IC50=591 nM | |||
| CHLA-10 | Growth inhibitory assay | 10 μM | IC50=102 nM | |||
| CHLA-258 | Growth inhibitory assay | 10 μM | IC50=1.05 μM | |||
| GBM2 | Growth inhibitory assay | 10 μM | IC50=9.15 μM | |||
| NB-1643 | Growth inhibitory assay | 10 μM | IC50=5.4 μM | |||
| NB-Ebc1 | Growth inhibitory assay | 10 μM | IC50>10 μM | |||
| CHLA-90 | Growth inhibitory assay | 10 μM | IC50>10 μM | |||
| CHLA-136 | Growth inhibitory assay | 10 μM | IC50>10 μM | |||
| NALM-6 | Growth inhibitory assay | 10 μM | IC50=265 nM | |||
| COG-LL-317 | Growth inhibitory assay | 10 μM | IC50=6.49 nM | |||
| RS4;11 | Growth inhibitory assay | 10 μM | IC50=147 nM | |||
| MOLT-4 | Growth inhibitory assay | 10 μM | IC50=40 nM | |||
| CCRF-CEM | Growth inhibitory assay | 10 μM | IC50=268 nM | |||
| Kasumi-1 | Growth inhibitory assay | 10 μM | IC50=107 nM | |||
| Karpas-299 | Growth inhibitory assay | 10 μM | IC50=2.93 μM | |||
| Ramos-RA1 | Growth inhibitory assay | 10 μM | IC50=7.35 μM | |||
| H1299 | Kinase assay | 10 μM | inhibits IKBKE-induced Akt Activation | |||
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 369.42 | Fórmula | C23H19N3O2 |
Almacenamiento (Desde la fecha de recepción) | |
|---|---|---|---|---|---|
| Nº CAS | 905854-02-6 | Descargar SDF | Almacenamiento de soluciones madre |
|
|
| Sinónimos | ARQ 197 | Smiles | C1CC2=C3C(=CC=C2)C(=CN3C1)C4C(C(=O)NC4=O)C5=CNC6=CC=CC=C65 | ||
Solubilidad
|
In vitro |
DMSO
: 73 mg/mL
(197.6 mM)
Ethanol : 35 mg/mL Water : Insoluble |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| Características |
The first selective c-Met inhibitor to be advanced into human clinical trials.
|
|---|---|
| Targets/IC50/Ki |
c-Met
(Cell-free assay) 0.355 μM(Ki)
|
| In vitro |
Se ha demostrado que ARQ-197 previene las respuestas celulares inducidas por HGF/c-met in vitro. Este compuesto posee actividad antitumoral; inhibiendo la proliferación de células A549, DBTRG y NCI-H441 con una IC50 de 0,38, 0,45, 0,29 μM. El tratamiento con este agente resulta en una disminución de la fosforilación de la cascada de señalización MAPK y la prevención de la invasión y migración. Además, la expresión ectópica de c-Met en NCI-H661, una línea celular sin expresión endógena de c-Met, hace que adquiera un fenotipo invasivo que también es suprimido por este químico. Aunque la adición de concentraciones crecientes de este inhibidor no afecta significativamente el Km de ATP, la exposición de c-Met a 0,5 μM de esta sustancia disminuyó la Vmax de c-Met en aproximadamente 3 veces. La capacidad de esta molécula para disminuir la Vmax sin afectar el Km de ATP confirmó que inhibe c-Met a través de un mecanismo no-ATP-competitivo y, por lo tanto, puede explicar su alto grado de selectividad de quinasa. Previene el c-Met recombinante humano con una constante inhibidora calculada Ki de aproximadamente 355 nM. Aunque la concentración más alta de ATP utilizada es de 200 μM, la potencia de este compuesto contra c-Met no se reduce al usar concentraciones de ATP de hasta 1 mM. Bloquea la fosforilación de c-Met y las vías de señalización de c-Met aguas abajo. Este químico suprime la autofosforilación constitutiva y mediada por ligando de c-Met y, por extensión, la actividad de c-Met, lo que a su vez conduce a la inhibición de los efectores de c-Met aguas abajo. Su inducción de apoptosis dependiente de caspasas se incrementa en células cancerosas humanas que expresan c-Met, incluidas las células HT29, MKN-45 y MDA-MB-231. |
| Ensayo de quinasa |
Ensayo de quinasa in vitro SDS-PAGE de c-Met
|
|
La proteína c-Met recombinante (100 ng) se preincuba con concentraciones crecientes de este compuesto durante 30 minutos a temperatura ambiente. Después de la preincubación, se añaden 100 μM de sustrato poli-Glu-Tyr y varias concentraciones de ATP que contienen 5 μCi de [γ-32P]ATP a la mezcla de reacción. La reacción se incuba durante 5 minutos a temperatura ambiente y luego se detiene con la adición de 5 μL de gel de SDS-poliacrilamida, tampón de muestra reductor. Las muestras se cargan luego en un gel de acrilamida al 7,5% y se realiza una SDS-PAGE. Los sustratos poli-Glu-Tyr fosforilados se visualizan finalmente mediante autorradiografía. La actividad de c-Met se cuantifica por densitometría.
|
|
| In vivo |
Los tres modelos de xenoinjertos tratados con Tivantinib muestran reducciones en el crecimiento tumoral: 66% en el modelo HT29, 45% en el modelo MKN-45 y 79% en el modelo MDA-MB-231. En estos estudios de xenoinjertos, no se observaron cambios significativos en el peso corporal después de la administración oral de este compuesto a 200 mg/kg. Farmacodinámicamente, la fosforilación de c-Met en tumores de xenoinjertos de colon humano (HT29) se inhibe fuertemente por este químico, según se evaluó por una reducción dramática de la autofosforilación de c-Met 24 horas después de una dosis oral única de 200 mg/kg de este agente. Esta misma dosis en ratones exhibe que los xenoinjertos tumorales están expuestos a niveles plasmáticos sostenidos del compuesto, consistente con la inhibición farmacodinámica observada de la fosforilación de c-Met y la inhibición de la proliferación de líneas celulares cancerosas que albergan c-Met. Los niveles plasmáticos del agente 10 horas después de la dosificación se determinaron en 1,3 μM, más de 3 veces por encima de la constante inhibidora bioquímica de esta sustancia para c-Met. Por lo tanto, es capaz de suprimir su objetivo in vivo en el tejido tumoral humano xenoinjertado. En conclusión, este inhibidor bloquea el crecimiento de tumores humanos xenoinjertados dependientes de c-Met. |
Referencias |
|
Aplicaciones
| Métodos | Biomarcadores | Imágenes | PMID |
|---|---|---|---|
| Western blot | cMET / p-cMET / p-AKT / p-ERK / p-rpS6 |
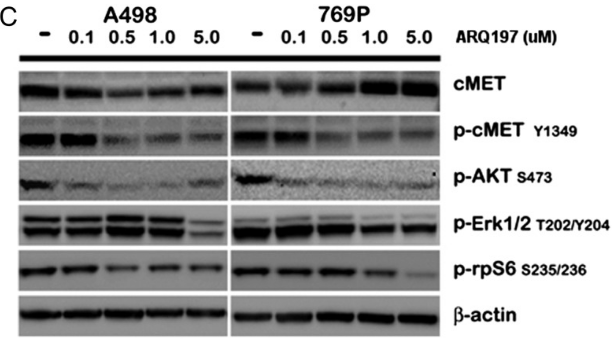
|
23022995 |
| Growth inhibition assay | Cell viability |
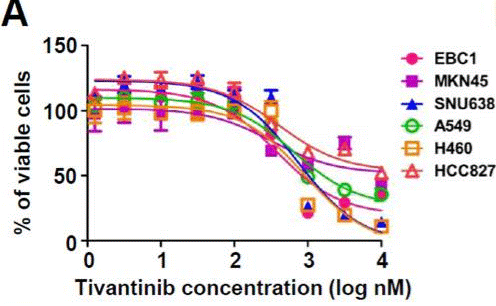
|
23598276 |
Información del ensayo clínico
(datos de https://clinicaltrials.gov, actualizado el 2024-05-22)
| Número NCT | Reclutamiento | Condiciones | Patrocinador/Colaboradores | Fecha de inicio | Fases |
|---|---|---|---|---|---|
| NCT02150733 | Completed | Hepatic Impairment|Solid Tumor|Cancer |
Daiichi Sankyo|Medpace Inc. |
April 2014 | Phase 1 |
| NCT01892527 | Completed | Colorectal Cancer Metastatic|C-met Overexpression |
Armando Santoro MD|Istituto Clinico Humanitas |
March 2013 | Phase 2 |
| NCT02049060 | Completed | Malignant Pleural Mesothelioma|Nonsquamous Nonsmall Cell Neoplasm of Lung |
Armando Santoro MD|Istituto Clinico Humanitas |
January 2013 | Phase 1|Phase 2 |
| NCT01755767 | Completed | Hepatocellular Carcinoma |
Daiichi Sankyo|ArQule Inc. a subsidiary of Merck Sharp & Dohme LLC a subsidiary of Merck & Co. Inc. (Rahway NJ USA) |
December 27 2012 | Phase 3 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
Preguntas frecuentes
Pregunta 1:
Are there any other solutions (apart from DMSO) I can dissolve it for in vivo experiment?
Respuesta:
S2753 This compound (ARQ 197) can be dissolved in 1% methylcellulose at 15 mg/ml as a suspension.
