solo para uso en investigación
3-Methyladenine (3-MA) Inhibidor de Autophagy/PI3K
Cat. No.S2767
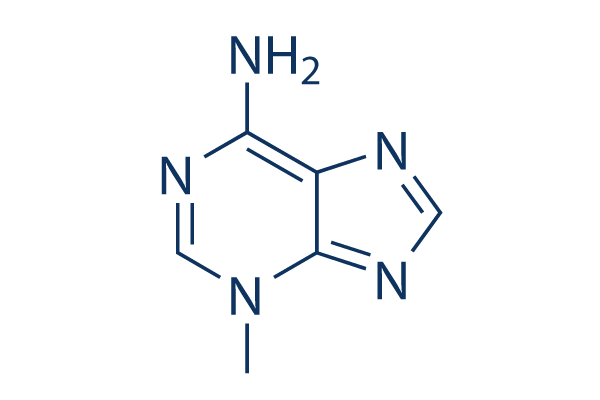
Estructura química
Peso molecular: 149.15
Control de calidad
Productos que se suelen utilizar junto con 3-Methyladenine (3-MA)
| Dianas relacionadas | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Otros PI3K Inhibidores | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| K562 | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| Jurkat | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| HeLa | Cytotoxicity Assay | 2mM | 24h | inhibites the cytotoxicity of silibinin to HeLa cells. | 21875385 | |
| PC12/TetOn | Function Assay | 0.1/1mM | 18h | leads to α-syn(WT) accumulation, toxicity, and oligomer formation | 21906659 | |
| RMPI8226 | Function Assay | 5mM | 1h | suppresses the level of autophagy under nutrient depletion | 21915620 | |
| MCF-7 | Function Assay | 10mM | 48h | blocks autophagy induced by bortezomib | 21931937 | |
| HBx | Apoptosis Assay | 10mM | 48h | DMSO | increases cell death | 22020078 |
| Marc-145 | Function Assay | 5mM | 12/24/36h | reduces the PRRSV titers and the protein expression | 22119900 | |
| U937 | Function Assay | 2mM | 12h | decreases the autophagy ratio | 22155150 | |
| BGC-823 | Function Assay | 5mM | 2h | inhibits the rate of autophagic cells | 22322152 | |
| A549 | Function Assay | 0.1mM | 24h | suppresses SU11274-induced cell death | 22466960 | |
| pDCs | Function Assay | 10mM | 0.5h | reduces the induction of IFN-α by ssRNA40 | 22396599 | |
| HeLa | Apoptosis Assay | 5mM | 24h | induces caspase-dependent cell death | 22545128 | |
| U251 | Apoptosis Assay | 5mM | 24h | increases S1-induced cell death | 22579788 | |
| MCF-7 | Apoptosis Assay | 0.1mM | 6h | enhances sirtinol-induced apoptosis | 22751989 | |
| PC-3 | Apoptosis Assay | 2mM | 2h | increases ORI-induced cell death | 22745580 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | DMSO | enhances apigenin-induced cell death | 24626522 |
| U2OS | Growth Inhibition Assay | 10mM | 24h | intensifies the growth inhibition induced by Dox | 24639013 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| HepG2 | Function Assay | 5mM | 4h | increases cellular levels of HL | 24713587 | |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| MDA-MB 231 | Apoptosis Assay | 5mM | 0.5h | modulates Tocomin® induced apoptosis | 24830781 | |
| PANC-1 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDA-MB-231 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MDA-MB-231 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| MCF-7 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MCF-7 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| HepG2 | Apoptosis Assay | 3mM | 5h | reduces cell apoptosis induced by QDs | 22836595 | |
| HeLa | Apoptosis Assay | 10mM | 2h | decreases cell viability co-treatment with PEI | 23000135 | |
| SK-HEP-1 | Apoptosis Assay | 10mM | 1h | protects against autophagy and induces apoptosis in bufalin-treated cells | 22858649 | |
| MDA-MB231 | Function Assay | 5mM | 1h | increases resveratrol-mediated caspase activation and cell death | 23088850 | |
| PaCa44 | Apoptosis Assay | 2.5mM | 1h | reduces genipin-mediated apoptosis | 23124112 | |
| T-47D | Function Assay | 10mM | 2h | inhibits autophagy process and increases rapamycin induced apoptosis | 23300026 | |
| GTL-16 | Apoptosis Assay | 5mM | 24h | reduces cell viability as compared to cells treated with MET inhibitors | 23313490 | |
| U251MG | Function Assay | 3mM | 1h | suppresses LC3-II protein expression | 23338618 | |
| T24 | Function Assay | 10mM | 1h | reduces the cleavage of LC3 after baicalin treatment | 23354080 | |
| HUVECs | Function Assay | 3mM | 24h | blocks the protective effect of resveratrol by inhibiting autophagy | 23358928 | |
| MCF-7 | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| Hela | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| OR6 | Function Assay | 10mM | 72h | suppresses HCV replication and formation of autophagosomes | 23395875 | |
| HT-29 | Function Assay | 1mM | 48/96h | inhibits AMPK induces autophagic cell death | 23508272 | |
| SH-SY5Y | Cytotoxicity Assay | 5mM | 24h | increases PCN toxicity | 23525265 | |
| Saos-2 | Apoptosis Assay | 1mM | 96h | increases cell death induced by PCX | 23563171 | |
| 1321N1 | Cytotoxicity Assay | 5mM | 24h | protects cell against PCN-induced toxicity | 23525265 | |
| A2780 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| OV2008 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| PC12 | Function Assay | 10mM | 24h | water | inhibits chymotrypsin-like proteasomal activity. | 23603979 |
| SH-SY5Y | Apoptosis Assay | 5mM | 1h | abolishes celastrol neuroprotective effect | 23619395 | |
| SH-SY5Y | Function Assay | 1mM | 24h | inhibits the autophagy induced by TOCP | 23743148 | |
| HepG2 | Function Assay | 10mM | 24h | inhibits siTIGAR- and HBSS-induced autophagy | 23817040 | |
| HeLa | Function Assay | 10mM | 2h | suppresses LC3 II expressison | 23864738 | |
| HONE-1 | Function Assay | 5mM | 1h | represses 6r-mediated ROS production | 23892358 | |
| MCF7 | Function Assay | 5mM | 24h | increases CuO induced cell death | 23962629 | |
| HO8910 | Apoptosis Assay | 10mM | 12h | enhances B19-induced apoptosi | 23983610 | |
| SMMC-7721 | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| Hep3B | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| H460 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| A549 | Function Assay | 10mM | 4h | inhibits autophagy induced by irradiation | 24142735 | |
| H1299 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| WiDr | Function Assay | 10mM | 1h | inhibits PCBL-induced LC3 II expression | 24190489 | |
| LoVo | Apoptosis Assay | 5mM | 48h | enhances DCA-induced apoptosis. | 24201812 | |
| HepG2 E47 | Function Assay | 2.5mM | 48h | increases the toxicity of AA, BSO, and CCl4 | 24273738 | |
| RKO | Function Assay | 2mM | 1h | DMSO | enhances cell death by geldanamycin | 24291777 |
| Hep3B | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| ACHN-5968 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| Huh7 | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| UOK257 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| ECSCs | Apoptosis Assay | 10mM | 4h | decreases rapamycin-treated apoptosis | 24319109 | |
| MCF-7 | Function Assay | 10mM | 24h | inhibits the autophagy induced by chemotherapy drugs | 24315578 | |
| SGC-7901 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| SMMC-7721 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| T24 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| NTUB1 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| MG-63 | Apoptosis Assay | 10mM | 12h | enhances DP-induced apoptosis | 24358301 | |
| MG-63 | Apoptosis Assay | 0.5/1mM | 32h | enhances salinomycin-induced cell apoptosis | 24358342 | |
| MG-63 | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| U2OS | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| HGC-27 | Function Assay | 10mM | 1h | inhibits the cell viability loss by RAD001 or MK-2206 | 24416349 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | enhances the apoptosis induced by apigenin | 24626522 | |
| A549 | Apoptosis Assay | 10mM | 48h | accelerates the reduction of cell viability induced by PTX | 24626722 | |
| Saos-2 | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| U2OS | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| HepG2 | Function Assay | 5mM | 4h | increases HL release | 24713587 | |
| A549 | Apoptosis Assay | 5mM | 48h | decreases the percentage of cell death and expression levels of caspase-3, Beclin-1 and LC3-II | 24706303 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| HT-29 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDR | Apoptosis Assay | 10mM | 6h | strengthens the power of anticancer drugs | 25019701 | |
| H157 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A549 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A2780cp | Growth Inhibition Assay | 1mM | 1h | increases cisplatin-induced cell death | 25322694 | |
| NBL-W-S | Apoptosis Assay | 1mM | 6h | increases cell apoptosis induced by GANT-61 | 25323222 | |
| NBL-W-S | Growth Inhibition Assay | 1mM | 6h | enhances GANT-61 toxicity | 25323222 | |
| A549 | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| 95D | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| A549 | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| 95D | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| Nara-H | Growth Inhibition Assay | 5mM | 48h | enhances temsirolimusmediated suppression of Nara-H cell proliferation | 21805033 | |
| HUVECs | Function Assay | 10mM | 0.5h | decreases the AGE-BSAinduced autophagy leve | 21468592 | |
| HepG2 | Apoptosis Assay | 2mM | 1h | enhances radiation-induced cell death | 21453691 | |
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 149.15 | Fórmula | C6H7N5 |
Almacenamiento (Desde la fecha de recepción) | 3 years -20°C powder |
|---|---|---|---|---|---|
| Nº CAS | 5142-23-4 | Descargar SDF | Almacenamiento de soluciones madre | Las soluciones son inestables. Preparar frescas o comprar tamaños pequeños preenvasados. Reenvasar al recibirlas. | |
| Sinónimos | NSC 66389 | Smiles | CN1C=NC(=N)C2=C1N=CN2 | ||
Solubilidad
|
In vitro |
DMSO
: 10 mg/mL
(67.04 mM)
Calentado con baño de agua a 50°C;
Ultrasonido;
Ethanol : 10 mg/mL Water : 4 mg/mL |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| Targets/IC50/Ki |
Autophagy
Vps34
(HeLa cells) 25 μM
PI3Kγ
(HeLa cells) 60 μM
|
|---|---|
| In vitro |
La ligera preferencia por la prevención de Vps34 por la 3-Methyladenine (3-MA) probablemente surge de un anillo hidrofóbico específico de Vps34, que rodea el grupo 3-metilo de este compuesto. Se ha informado que causa la muerte de células cancerosas tanto en condiciones normales como de inanición, y también podría suprimir la migración e invasión celular independientemente de su capacidad para inhibir la Autophagy, lo que implica que posee funciones distintas de la supresión de la Autophagy. Este compuesto provoca la muerte celular dependiente de caspasa que es independiente de la inhibición de la Autophagy. El tratamiento con 5 mM de este compuesto reduce el porcentaje de células HeLa privadas de glucosa que muestran puntos GFP-LC3 al 23%. Los niveles de LC3-I aumentan y los niveles de LC3-II disminuyen entre 12 y 48 horas en células tratadas con 3-MA. La conversión de LC3-I a LC3-II es suprimida por el compuesto. El tratamiento de células HeLa con 2,5 mM o 5 mM durante un día no afecta la viabilidad celular, mientras que el tratamiento con 10 mM durante un día causa una disminución del 25,0% en la viabilidad celular. El tratamiento de células con 2,5, 5 o 10 mM durante dos días causa una disminución del 11,5%, 38,0% y 79,4% en la viabilidad, respectivamente. Disminuye la viabilidad celular de forma dependiente del tiempo y la dosis, y acorta significativamente la duración de la detención de la prometafase inducida por nocodazol. La supresión de la Autophagy por 3-MA inhibe la muerte celular inducida por SU11274. El tratamiento prolongado con este compuesto (hasta 9 horas) induce una conversión significativa de LC3 I a II en MEF de tipo salvaje. El tratamiento prolongado con 3-MA, pero no con wortmannin, aumenta notablemente la puntuación/agregación de GFP-LC3. Su conversión de LC3 inducida y la liberación de GFP libre son dependientes de ATG7. El tratamiento con este compuesto conduce a un aumento evidente del nivel de proteína p62. El compuesto aumenta el nivel de p62 incluso en MEF Atg5-/-- así como en células con deleción de ATG5 mediada por DOX. Inhibe la PI3K de clase I y clase III en diferentes patrones temporales. Su conversión de LC3 I a LC3 II inducida está dramáticamente comprometida en células Tsc2-/-- en comparación con células de tipo salvaje. Este compuesto interrumpe la función anti-autofágica del complejo mTOR 1. |
| Ensayo de quinasa |
Ensayo de degradación de proteínas
|
|
Las células HeLa se radiomarcan durante 24 horas con 0,05 mCi/mL de l-[U- 14C]valina. Al final del período de marcado, las células se enjuagan tres veces con PBS. Las células se incuban durante los tiempos designados en medio completo o EBSS con o sin la presencia de 10 mM de 3-Methyladenine (3-MA).
|
|
| In vivo |
La 3-Methyladenine (3-MA) bloquea la Autophagy a través de su efecto sobre la fosfatidilinositol 3-cinasa (PI3K) de clase III. El tratamiento con este compuesto no altera el grado de hemorragia en comparación con el grupo de hemorragia subaracnoidea (HSA). Su pretratamiento agrava significativamente los síntomas neurológicos en comparación con el grupo HSA + vehículo. La Autophagy disminuye cuando se aplica. Por el contrario, la caspasa-3 escindida está marcadamente regulada al alza en el grupo HSA + 3-MA. En línea con la regulación al alza de la expresión de la caspasa-3 escindida, el número de células TUNEL-positivas en el córtex derecho aumenta significativamente en el grupo HSA + 3-MA en comparación con el grupo HSA + vehículo. |
Referencias |
|
Aplicaciones
| Métodos | Biomarcadores | Imágenes | PMID |
|---|---|---|---|
| Western blot | α-SMA / TGF-β / LC-3BI / LC-3B II / Beclin-1 / NF-κB p65 caspase-3 / caspase-9 / PARP VEGF APP / BACE1 / ADAM17 / Presenilin 1 / Presenilin 2 / Nicastrin / APH-1 / Pen-2 / LC3-1 / LC3-2 |
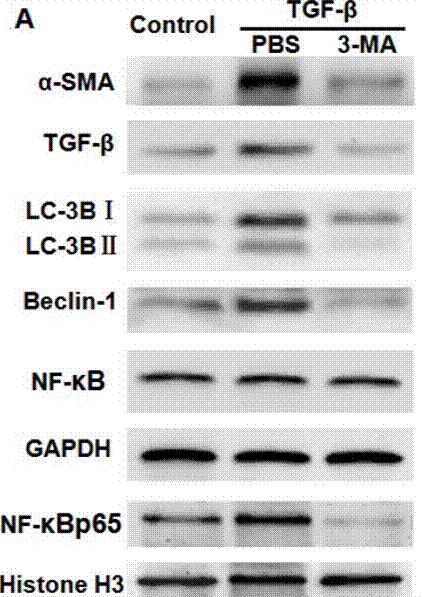
|
29296191 |
| Immunofluorescence | LC3 / Hif-α / COX2 |
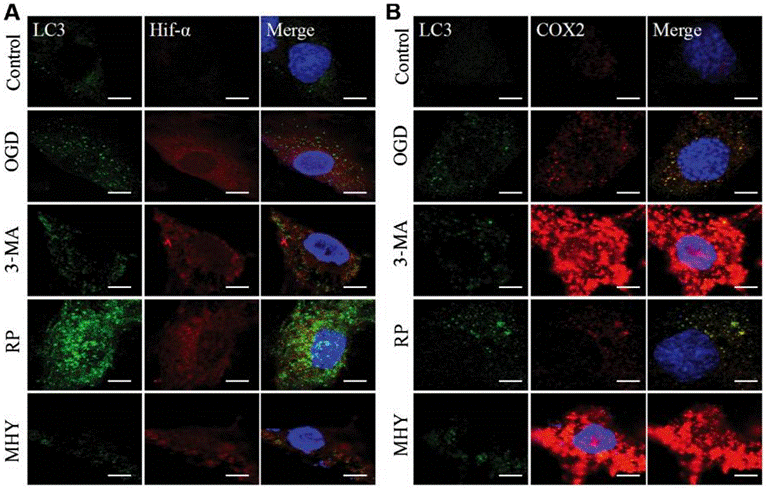
|
29039446 |
| Growth inhibition assay | Cell viability |
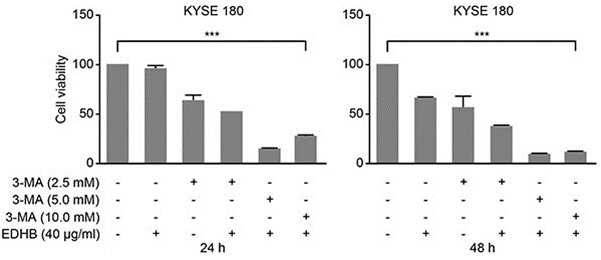
|
26934124 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
Preguntas frecuentes
Pregunta 1:
I'm also wondering whether it can be dissolved in water, or maybe something like culture medium, normal saline solution to form 10mM solution.
Respuesta:
As the reference (http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal. pone.0035665), it was found to inhibit autophagy at concentrations ranging from 1 to 10 mM and was directly dissolved into the culture medium at the indicated concentrations. And we tested the solubility of S2767, and found its solubility in DMEM is 31 mg/mL at about 40°C.
