solo para uso en investigación
Tenofovir Disoproxil Fumarate Reverse Transcriptase inhibidor
Cat. No.S1400
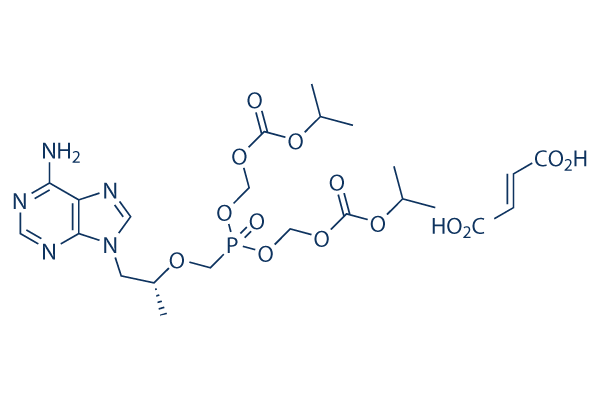
Estructura química
Peso molecular: 635.51
Control de calidad
| Dianas relacionadas | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite HIV HCV Protease |
|---|---|
| Otros Reverse Transcriptase Inhibidores | Dapivirine (TMC120) Fangchinoline Salicylanilide 3'-Fluoro-3'-deoxythymidine (Alovudine) Ulonivirine Lersivirine (UK-453061) Bifendate 4-Chloro-2-(trifluoroacetyl)aniline hydrochloride |
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| MT2 | Function assay | 5 days | Inhibition of virus-induced cytopathic effect in wild type HIV 3a infected MT2 cells after 5 days, EC50=0.015μM | 17562366 | ||
| HepG2 | Cytotoxicity assay | 9 days | Cytotoxicity against human HepG2 cells after 9 days by MTT assay, IC50=2.31μM | 17888662 | ||
| HepG2 | Antiviral assay | 9 days | Antiviral activity against Hepatitis B virus infected human HepG2 cells after 9 days by MTT assay, IC50=5.1μM | 17888662 | ||
| MT-2 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT-2 cells by two fold dilution method in presence of 10% FBS, EC50=0.0068μM | 19104010 | |||
| MT2 | Antiviral assay | Antiviral activity against HIV1 infected in human MT2 cells assessed as inhibition of viral replication, IC50=0.54μM | 19596885 | |||
| HeLa P4/R5 | Antiviral assay | Antiviral activity against HIV1 infected in human HeLa P4/R5 cells assessed as inhibition of viral replication, IC50=4.7μM | 19596885 | |||
| HeLa P4/R5 | Antiviral assay | Antiviral activity against HIV1 harboring reverse transcriptase K65R mutant infected in human HeLa P4/R5 cells assessed as inhibition of viral replication, IC50=11.4μM | 19596885 | |||
| human bone marrow cells | Cytotoxicity assay | 24 hrs | Cytotoxicity against human bone marrow cells after 24 hrs by BFU-E assay, CC50=0.9μM | 20439609 | ||
| human bone marrow cells | Cytotoxicity assay | 24 hrs | Cytotoxicity against human bone marrow cells after 24 hrs by GM-CFU assay, CC50=1.9μM | 20439609 | ||
| HeLa-T4 | Antiviral assay | 48 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef infected in human HeLa-T4 cells assessed as luciferase activity after 48 hrs by exogenous RT assay, EC50=0.0029μM | 21060108 | ||
| HeLa-T4 | Antiviral assay | 48 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef infected in human HeLa-T4 cells assessed as luciferase activity after 48 hrs by exogenous RT assay, EC95=0.037μM | 21060108 | ||
| HeLaT4 | Antiviral assay | 24 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of infection using human HeLaT4 cells pretreated for 24 hrs followed by exposed to vi, EC50=0.12μM | 21060108 | ||
| HeLaT4 | Antiviral assay | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of 2 mins magnetic nanopartials-medated infection in human HeLaT4 cells treated for 1, EC99.6=0.95μM | 21060108 | |||
| HeLaT4 | Cytotoxicity assay | Cytotoxicity against human HeLaT4 cells by WST-1 assay, CC50=34μM | 21060108 | |||
| HeLaT4 | Function assay | 24 hrs | Drug uptake in human HeLaT4 cells assessed as compound persist measured after 3 times washout at 100 time EC95 for HIV1 for 24 hrs | 21060108 | ||
| HeLaT4 | Antiviral assay | 24 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of infection using human HeLaT4 cells pretreated at 100 time EC95 for 24 hrs followed | 21060108 | ||
| PBMC | Antiviral assay | 7 days | Antiviral activity against HIV1 infected in human PBMC assessed as inhibition of viral replication by measuring reverse transcriptase activity in cell supernatant preincubated with cells followed by viral infection measured after 7 days by radioactive inc, EC50=0.0046μM | 27405794 | ||
| HepG2.2.15 | Antiviral assay | 3 days | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as inhibition of viral DNA in cell supernatant incubated for 3 days in presence of 10% FBS followed by compound treatment in absence of 10% FBS for 3 days by qRT-PCR method, EC50=0.34μM | 27405794 | ||
| HepG2.2.15 | Cytotoxicity assay | 6 days | Cytotoxicity against human HepG2.2.15 cells assessed as reduction in cell viability after 6 days by XTT assay, CC50=29.2μM | 27405794 | ||
| PBMC | Antiviral assay | 30 mins | Antiviral activity against CXCR4-tropic HIV-1 NL4-3 infected in human PHA-stimulated PBMC assessed as inhibition of viral replication by measuring reduction in p24 antigen production preincubated with cells for 30 mins followed by viral infection measured, IC50=0.08μM | 28682067 | ||
| PBMC | Antiviral assay | 30 mins | Antiviral activity against CCR5 tropic HIV1 BaL infected in human PHA-stimulated PBMC assessed as inhibition of viral replication by measuring reduction in p24 antigen production preincubated with cells for 30 mins followed by viral infection measured on , IC50=0.22μM | 28682067 | ||
| MT4 | Antiviral assay | 6 days | Antiviral activity against CXCR4-tropic HIV-1 NL4-3 infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 6 days by XTT dye based assay, EC50=4.89μM | 28682067 | ||
| MT4 | Antiviral assay | 6 days | Antiviral activity against tenofovir-resistant CXCR4-tropic HIV-1 NL4-3 harboring reverse transcriptase K65R mutant infected in human MT4 cells assessed as inhibition of viral replication inhibition of virus-induced cytopathic effect after 6 days by XTT d, EC50=11.3μM | 28682067 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as reduction in cytoplasmic DNA synthesis by reed and munch method, IC50=0.85μM | 31223460 | |||
| HepG2.2.15 | Cytotoxicity assay | Cytotoxicity against human HepG2.2.15 cells infected with HBV, CC50=20.71μM | 31223460 | |||
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 635.51 | Fórmula | C19H30N5O10P.C4H4O4 |
Almacenamiento (Desde la fecha de recepción) | |
|---|---|---|---|---|---|
| Nº CAS | 202138-50-9 | Descargar SDF | Almacenamiento de soluciones madre |
|
|
| Sinónimos | GS 1278, Tenofovir DF | Smiles | CC(C)OC(=O)OCOP(=O)(COC(C)CN1C=NC2=C(N=CN=C21)N)OCOC(=O)OC(C)C.C(=CC(=O)O)C(=O)O | ||
Solubilidad
|
In vitro |
DMSO
: 100 mg/mL
(157.35 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| Targets/IC50/Ki |
HIV reverse transcriptase
(Cell-free assay) |
|---|---|
| In vitro |
El tenofovir se elimina de la circulación sistémica por vía renal a través de una combinación de filtración glomerular y secreción tubular activa. El tenofovir no es un sustrato para el transportador de cationes orgánicos humanos tipo 1 (hOCT1) o hOCT2. El tenofovir se acumula a niveles cinco veces menores en las células que sobreexpresan MRP4, y su acumulación podría aumentar con un inhibidor de MRP. El tenofovir no produce cambios significativos en los niveles de ADN mitocondrial (ADNmt) en células de hepatoblastoma humano (HepG2), células de músculo esquelético (SkMCs) o células epiteliales de túbulo proximal renal. El tenofovir eleva la producción de lactato en menos del 20% en células HepG2 o SkMCs. El tenofovir se fosforila eficientemente a difosfato de tenofovir (TFV-DP) tanto en células HepG2 como en hepatocitos humanos primarios. El tenofovir tiene una concentración efectiva del 50% de 1.1 mM contra el VHB en ensayos basados en células, y la potencia mejora más de 50 veces con la adición de progrupos de bis-isoproxil. El tenofovir ha demostrado previamente actividad completa contra el VHB resistente a la lamivudina in vitro y clínicamente. El tenofovir inhibe la proliferación de células HepG2 derivadas del hígado y células musculares esqueléticas normales con valores de CC(50) de 398 μM y 870 μM, respectivamente. El tenofovir muestra efectos sustancialmente más débiles sobre la proliferación y viabilidad de las células epiteliales del túbulo proximal renal que el cidofovir, un análogo nucleotídico relacionado con el potencial de inducir disfunción tubular renal. |
Referencias |
|
Información del ensayo clínico
(datos de https://clinicaltrials.gov, actualizado el 2024-05-22)
| Número NCT | Reclutamiento | Condiciones | Patrocinador/Colaboradores | Fecha de inicio | Fases |
|---|---|---|---|---|---|
| NCT05874440 | Recruiting | Chronic Hepatitis b Patients |
Sohag University |
April 15 2023 | -- |
| NCT03576066 | Completed | Chronic Hepatitis B |
Assembly Biosciences |
June 11 2018 | Phase 2 |
| NCT03361956 | Completed | Hepatitis B |
Janssen Sciences Ireland UC |
February 13 2018 | Phase 2 |
| NCT02985996 | Completed | HIV Infections |
Emory University|Centers for Disease Control and Prevention |
February 6 2017 | Phase 1 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
