solo para uso en investigación
Acyclovir (Aciclovir) Antiviral químico
Cat. No.S1807
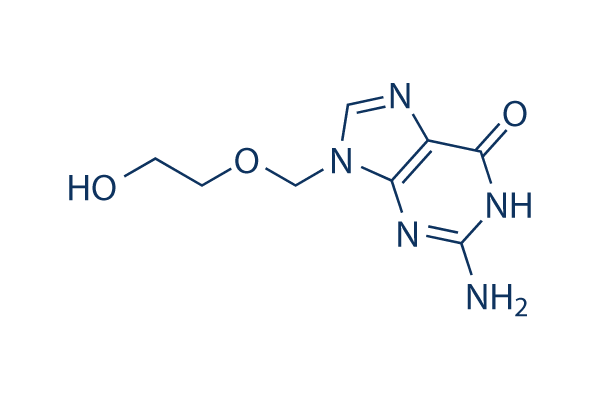
Estructura química
Peso molecular: 225.2
Control de calidad
| Dianas relacionadas | Integrase Bacterial Antibiotics Anti-infection Fungal COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Otros Antiviral Inhibidores | Moroxydine HCl Aloperine GS-441524 Oleanolic Acid Harringtonine NGI-1 U18666A Aloin B Saikosaponin B2 Lapachol |
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| BSC-1 cells | Function assay | Antiviral activity of the compound was evaluated against the Herpes simplex virus type-1 in BSC-1 cells, IC50=2.6 μM | ||||
| P3HR-1 cells | Function assay | Effective concentration for the inhibition of Epstein-Barr virus EBV-DNA synthesis in human lymphoblastoid P3HR-1 cells, EC50=6.75 μM | ||||
| HFF cells | Function assay | Concentration for HSV-1 plaque reduction (VPR) by 50% in HFF cells, EC50=1.1 μM | ||||
| HFF cells | Function assay | Inhibitory concentration required to reduce HSV-1 induced cytopathogenic effect (CPE) by 50 % in HFF cells, EC50=2.22 μM | ||||
| human embryonic lung cells | Function assay | Antiviral activity was measured as effective concentration required to reduce Varicella Zoster virus (OKA)-induced plaque formation in human embryonic lung cells, EC50=1.1 μM | ||||
| HEL cells | Function assay | Compound was tested for anti-viral activity against HSV-1(G) in HEL cells, EC50=1.3 μM | ||||
| HEL cell | Function assay | Effective concentration required to inhibit Tyrosine kinase (TK+) Varicella-Zoster virus-induced cytopathicity by 50% in OKA strain HEL cell lines, EC50=4.53 μM | ||||
| HSV-2 MS Vero cells | Function assay | Inhibition of viral cytopathic effect in infected human foreskin fibroblast cell monolayers of HSV-2 MS Vero cells by 50%, EC50=6.2 μM | ||||
| HSV-1 E-377 Vero cells | Function assay | Inhibition of plaque formation in monolayers of HSV-1 E-377 Vero cells by 50%, EC50=4.4 μM | ||||
| HSV-1 KOS Vero cells | Function assay | Inhibition of plaque formation in monolayers of HSV-1 KOS Vero cells by 50%, EC50=2.2 μM | ||||
| HeLa cell | Function assay | Ability to inhibit cytopathogenicity of herpes simplex type 1 virus (G) in HeLa cell culture, IC50=0.19 μM | ||||
| HEp-2 cells | Function assay | Minimum inhibitory concentration causing 25% inhibition of cytopathic effect induced by Herpes simplex virus-1 (K979) in HEp-2 cells | ||||
| HeLa cells | Function assay | Inhibition of HSV-1 DNA synthesis in virus-infected HeLa cells, IC50=1.9 μM | ||||
| human HFF cells | Function assay | Inhibitory concentration of the drug against the cytopathic effect for E-377 strain of herpes simplex virus-1 (HSV-1) in human HFF cells, EC50=0.04 μM | ||||
| human HFF cells | Function assay | Inhibitory concentration of the drug against the cytopathic effect for MS strain of herpes simplex virus-2 (HSV-2) in human HFF cells, EC50=0.09 μM | ||||
| MRC-5 cells | Function assay | Antiviral activity in plaque reduction assay was determined against herpes simplex virus type 2 (HSV-2) in MRC-5 cells, IC50=2.5 μM | ||||
| human lung fibroblasts (MRC-5) | Function assay | Compound was tested for antiviral activity against Herpes Simplex virus Type-1(18189) in human lung fibroblasts (MRC-5) | ||||
| Raji cells | Function assay | Inhibitory concentration of the drug against the antigen production against P3HR-1 strain of epstein barr virus-2 (EBV) in Raji cells, EC50=2.9 μM | ||||
| Vero cells | Function assay | Inhibitory activity against herpes simplex virus type 2 (HSV 2) strain 186 in Vero cells, IC50=1.7 μM | ||||
| BSC-1 cells | Function assay | Effective concentration required to inhibit herpes simplex virus 1 in BSC-1 cells in ELISA, EC50=1.5 μM | ||||
| HFF cells | Function assay | Effective concentration required to inhibit varicella zoster virus replication in HFF cells, EC50=1.6 μM | ||||
| HFF cells | Function assay | Effective concentration required to inhibit herpes simplex virus 1 in HFF cells in cytopathic effect (CPE) assay, EC50=0.9 μM | ||||
| Daudi cells | Function assay | Inhibition of EBV replication in Daudi cells by viral capsid antigen-ELISA, EC50=0.33 μM | ||||
| african green monkey Vero cells | Function assay | 48 h | Antiviral activity against HSV2 strain 333 infected in african green monkey Vero cells assessed as inhibition of cytopathic effect after 48 hrs by plaque reduction assay, EC50=1.87 μM | |||
| WI-38 cell | Function assay | Anti viral activity against VZV(pplla strain) in WI-38 cell monolayers, ID50=4 μM | ||||
| HEL cells | Function assay | 4 days | Antiviral activity against HSV1 KOS infected in HEL cells assessed as inhibition of virus-induced cytopathogenicity after 4 days, EC50=0.2 μM | |||
| HEL cells | Function assay | Antiviral activity against HSV1 KOS infected in HEL cells assessed as inhibition of virus-induced cytopathic effect, EC50=0.14 μM | ||||
| African green monkey Vero 76 cells | Cytotoxicity assay | 48-96 h | Cytotoxicity against African green monkey Vero 76 cells assessed as cell viability after 48 to 96 hrs by crystal violet staining, CC50=13 μM | |||
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 225.2 | Fórmula | C8H11N5O3 |
Almacenamiento (Desde la fecha de recepción) | |
|---|---|---|---|---|---|
| Nº CAS | 59277-89-3 | Descargar SDF | Almacenamiento de soluciones madre |
|
|
| Sinónimos | Acycloguanosine, ACV, NSC 645011,BW 248U | Smiles | C1=NC2=C(N1COCCO)N=C(NC2=O)N | ||
Solubilidad
|
In vitro |
DMSO
: 45 mg/mL
(199.82 mM)
Water : Insoluble Ethanol : Insoluble |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| Targets/IC50/Ki |
HSV
|
|---|---|
| In vitro |
En un ensayo de reducción de placa en células Vero, se determina la sensibilidad al Acyclovir (Aciclovir) de los aislados del virus del herpes simple. Los valores de IC50 son consistentemente 2-3 veces más bajos en B2 en comparación con la cepa H de células Vero. Las cepas de HSV Tipo 2 son 2-10 veces menos sensibles que las cepas de Tipo 1. |
| In vivo |
Acyclovir (Aciclovir) oral en dosis bajas puede ser eficaz en la prevención de la infección por HSV durante el tratamiento con OKT3 de pacientes seropositivos. La continuación de este compuesto durante dos a cuatro semanas después de la finalización de la terapia con OKT3 puede prevenir la aparición de infecciones tardías. |
Referencias |
|
Información del ensayo clínico
(datos de https://clinicaltrials.gov, actualizado el 2024-05-22)
| Número NCT | Reclutamiento | Condiciones | Patrocinador/Colaboradores | Fecha de inicio | Fases |
|---|---|---|---|---|---|
| NCT06228430 | Not yet recruiting | Healthy Volunteer |
International Bio service |
February 12 2024 | Phase 1 |
| NCT05589688 | Not yet recruiting | Obesity |
University Hospital Toulouse |
January 2024 | Phase 1 |
| NCT06058858 | Not yet recruiting | Cytomegalovirus Infections|Acute Leukemia|B Cell Lymphoma |
Assistance Publique - Hôpitaux de Paris |
October 1 2023 | -- |
| NCT05468619 | Recruiting | Herpes Simplex |
National Institute of Allergy and Infectious Diseases (NIAID) |
September 23 2022 | Phase 1 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
