solo para uso en investigación
Doxycycline MMP Inhibitor
Cat. No.S5159
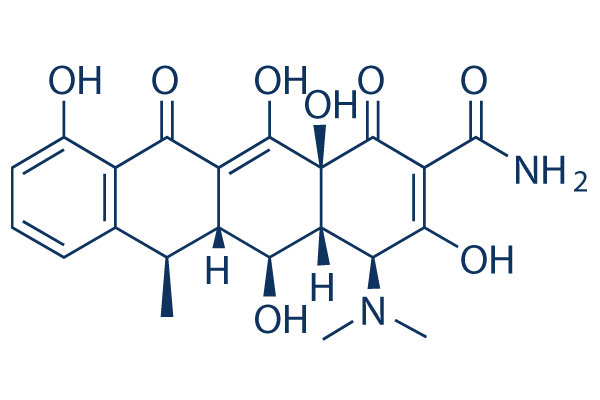
Estructura química
Peso molecular: 444.43
Control de calidad
- Citado en Nature Medicine por su calidad de primer nivel
- COA
- Ficha técnica
- SDS
- Citado en Nature Medicine por su calidad de primer nivel
- COA
- Ficha técnica
- SDS
- Citado en Nature Medicine por su calidad de primer nivel
- COA
- Ficha técnica
- SDS
- Citado en Nature Medicine por su calidad de primer nivel
- COA
- Ficha técnica
- SDS
- Citado en Nature Medicine por su calidad de primer nivel
- COA
- Ficha técnica
- SDS
| Dianas relacionadas | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Otros Antineoplastic and Immunosuppressive Antibiotics Inhibidores | Staurosporine (STS) Cyclosporin A Oligomycin A (MCH 32) Puromycin Dihydrochloride Nigericin sodium salt Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Sodium Monensin (NSC 343257) Cephalomannine |
Cultivo celular, tratamiento y concentración de trabajo
| Líneas celulares | Tipo de ensayo | Concentración | Tiempo de incubación | Formulación | Descripción de la actividad | PMID |
|---|---|---|---|---|---|---|
| Staphylococcus aureus 2 planktonic cells | Bactericidal assay | 24 hrs | Bactericidal activity against methicillin-resistant Staphylococcus aureus 2 planktonic cells after 24 hrs by calgary biofilm device method, MBC=2μM. | 29638121 | ||
| THP1 | Function assay | Selectivity index, ratio of IC50 for human THP1 cells to IC50 for Plasmodium falciparum, IC50=3.1μM. | 19748781 | |||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19482476 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19926173 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19482476 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19926173 | ||
| THP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human THP1 cells after 72 hrs by propidium iodide staining-based flow cytometry, IC50=20μM. | 19748781 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21741131 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 22889559 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21852132 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 24946216 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as cell viability after 72 hrs by MTT assay, CC50=20μM. | 25791675 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by MTT assay, CC50=20μM. | 25282267 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells measured after 72 hrs by MTT assay, CC50=20μM. | 27155463 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 72 hrs by MTT assay, CC50=20μM. | 27654395 | ||
| SW1353 | Function assay | 50 uM | Inhibition of IL-1-beta-induced MMP13 production in human SW1353 cells at 50 uM | 17267227 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against yafQ gene-deficient Escherichia coli K-12 BW25113 biofilm assessed as log reduction of viable cells after 24 hrs | 19307375 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against Escherichia coli K-12 BW25113 biofilm harboring pCA24N ptac::yafQ plasmid assessed as log reduction of viable cells after 24 hrs pretreated with 5 uM of IPTG for 4 hrs | 19307375 | ||
| Neuro2a | Function assay | 1 uM | Decrease in 7-DHC levels in Dhcr7-deficient mouse Neuro2a cells at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| vascular endothelial cells | Antibacterial assay | 25 ug/ml | 72 hrs | Antibacterial activity against Rickettsia prowazekii str. Breinl infected in CD rat primary pulmonary vascular endothelial cells assessed as bacterial shape change at 25 ug/ml measured 72 hrs post infection by Hoechst 33258/phalloidin staining based fluor | 28089350 | |
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Hep2 | Anti-Chlamydial assay | 1 ug/ml | 8 hrs | Anti-Chlamydial activity against Chlamydia trachomatis Serovar LGV-L2 infected in Hep2 cells assessed as reduction in size and number of chlamydial inclusion at 1 ug/ml treated at 8 hrs post-infection and 24 hrs later re-infecting fresh Hep2 cells monolay | 32227948 | |
| skeletal myoblast cells | Cytotoxicity assay | DNDI: Cytotoxicity in Vitro, 72 hour, in rat skeletal myoblast cells, IC50=14.49μM. | ChEMBL | |||
| Haga clic para ver más datos experimentales de líneas celulares | ||||||
Información química, almacenamiento y estabilidad
| Peso molecular | 444.43 | Fórmula | C22H24N2O8 |
Almacenamiento (Desde la fecha de recepción) | |
|---|---|---|---|---|---|
| Nº CAS | 564-25-0 | Descargar SDF | Almacenamiento de soluciones madre |
|
|
| Sinónimos | Vibramycin, Doxytetracycline, Doxiciclina, Doxycyclinum | Smiles | CC1C2C(C3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | ||
Solubilidad
|
In vitro |
DMSO
: 89 mg/mL
(200.25 mM)
Water : Insoluble Ethanol : Insoluble |
Calculadora de Molaridad
|
In vivo |
|||||
Calculadora de formulación in vivo (Solución clara)
Paso 1: Introduzca la información a continuación (Recomendado: Un animal adicional para tener en cuenta la pérdida durante el experimento)
Paso 2: Introduzca la formulación in vivo (Esto es solo la calculadora, no la formulación. Por favor, contáctenos primero si no hay una formulación in vivo en la sección de Solubilidad.)
Resultados del cálculo:
Concentración de trabajo: mg/ml;
Método para preparar el líquido maestro de DMSO: mg fármaco predissuelto en μL DMSO ( Concentración del líquido maestro mg/mL, Por favor, contáctenos primero si la concentración excede la solubilidad del DMSO del lote del fármaco. )
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadirμL PEG300, mezclar y clarificar, luego añadirμL Tween 80, mezclar y clarificar, luego añadir μL ddH2O, mezclar y clarificar.
Método para preparar la formulación in vivo: Tomar μL DMSO líquido maestro, luego añadir μL Aceite de maíz, mezclar y clarificar.
Nota: 1. Por favor, asegúrese de que el líquido esté claro antes de añadir el siguiente disolvente.
2. Asegúrese de añadir el (los) disolvente(s) en orden. Debe asegurarse de que la solución obtenida, en la adición anterior, sea una solución clara antes de proceder a añadir el siguiente disolvente. Se pueden utilizar métodos físicos como el vórtice, el ultrasonido o el baño de agua caliente para ayudar a la disolución.
Mecanismo de acción
| In vitro |
100 ng/mL-5 µg/mL Doxycycline puede alterar significativamente el perfil metabólico de la célula, así como reducir la tasa proliferativa, aunque el tamaño del efecto depende de la línea celular particular utilizada. Este compuesto detiene las células en la fase G1-S del ciclo celular en células PANC-1 e induce la expresión de p53 y su objetivo p21. Regula a la baja los genes antiapoptóticos e induce los genes proapoptóticos. Este químico también induce la apoptosis a través de una vía dependiente de Fas/Fas-ligando en linfocitos T Jurkat. |
|---|---|
| In vivo |
Doxycycline reduce con éxito el crecimiento tumoral in vivo (inhibió el crecimiento de células de cáncer de páncreas). |
Referencias |
|
Aplicaciones
| Métodos | Biomarcadores | Imágenes | PMID |
|---|---|---|---|
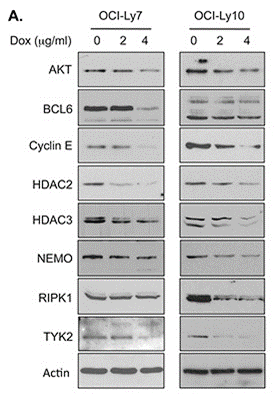
| Western blot | AKT / BCL6 / Cyclin E / HDAC2 / HDAC3 / NEMO / TYK2 / RIPK1 HSP70 / HSP90 pATM / ATM |
|
26142707 |
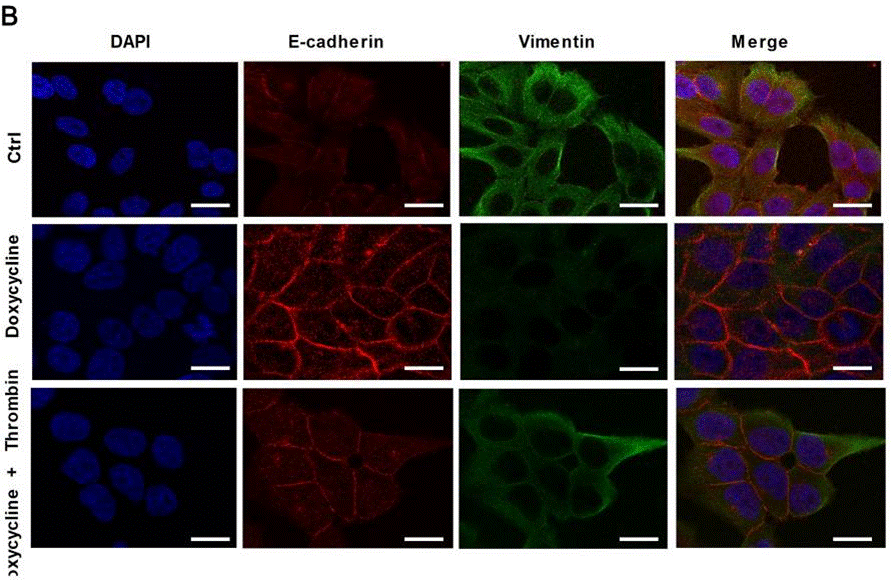
| Immunofluorescence | E-cadherin / Vimentin |
|
29285218 |
Información del ensayo clínico
(datos de https://clinicaltrials.gov, actualizado el 2024-05-22)
| Número NCT | Reclutamiento | Condiciones | Patrocinador/Colaboradores | Fecha de inicio | Fases |
|---|---|---|---|---|---|
| NCT05972772 | Not yet recruiting | Infectious Disease|Therapeutics |
Lao-Oxford-Mahosot Hospital Wellcome Trust Research Unit|Mahidol Oxford Tropical Medicine Research Unit |
March 20 2024 | Phase 2|Phase 3 |
| NCT06007534 | Recruiting | Post-exposure Prophylaxis|Sexually Transmitted Diseases|Doxycycline |
Assistance Publique - Hôpitaux de Paris |
October 25 2023 | Not Applicable |
| NCT04762134 | Recruiting | Bacterial Sexually Transmitted Diseases |
Jonathan Troy Grennan|Canadian Institutes of Health Research (CIHR)|British Columbia Centre for Disease Control |
June 2 2023 | Phase 4 |
| NCT05853120 | Recruiting | Sexually Transmitted Diseases |
Emory University|Centers for Disease Control and Prevention |
May 31 2023 | Phase 4 |
| NCT05382208 | Recruiting | Emphysema|HIV |
Weill Medical College of Cornell University|National Heart Lung and Blood Institute (NHLBI)|University of California Los Angeles|University of Iowa|University of Michigan |
August 22 2022 | Phase 2 |
| NCT05492019 | Recruiting | Parkinson Disease |
Bangabandhu Sheikh Mujib Medical University Dhaka Bangladesh |
July 1 2022 | Phase 2 |
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.
