Solo para uso en investigación
ATP citrate lyase Antibody [N17N8]
Nº de cat.: F4744
Aplicación:
Reactividad:
Fundamentos del experimento
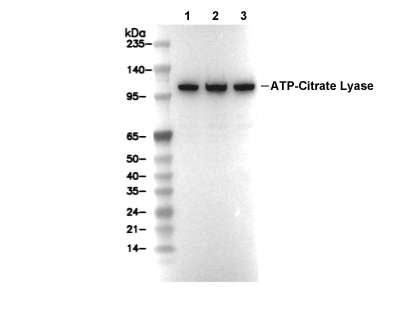
WB
Recommended SDS-PAGE separating gel concentration: 5%.
Recommended SDS-PAGE separating gel concentration: 5%.
Información de uso
| Dilución |
|---|
|
| Aplicación |
|---|
| WB |
| Reactividad |
|---|
| Human, Mouse, Rat |
| Fuente |
|---|
| Rabbit Monoclonal Antibody |
| Tampón de almacenamiento |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Almacenamiento (desde la fecha de recepción) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| PM predicho |
|---|
| 125 kDa |
| Control positivo | MCF7 cells; HeLa cells; Hep G2 cells; mIMCD-3 cells |
|---|---|
| Control negativo |
Métodos experimentales
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 5%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:1000), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
Descripción biológica
| Especificidad |
|---|
| ATP citrate lyase Antibody [N17N8] detects endogenous levels of total ATP citrate lyase protein. |
| Localización subcelular |
|---|
| Cytoplasm |
| Uniprot ID |
|---|
| P53396 |
| Clon |
|---|
| N17N8 |
| Sinónimo |
|---|
| ACLY; ATP-citrate synthase; EC:2.3.3.8; ATP-citrate (pro-S-)-lyase (ACL); Citrate cleavage enzyme |
| Antecedentes |
|---|
| ATP citrate lyase (ACLY), a cytosolic homotetrameric enzyme at the interface of glucose and lipid metabolism, cleaves citrate derived from mitochondrial export into acetyl-CoA and oxaloacetate to fuel de novo lipogenesis, cholesterol synthesis, and histone acetylation, organized into two major domains per subunit, an N-terminal citrate synthase-homology (CSH) domain encompassing residues ~1-700 that binds citrate and CoA to form citryl-CoA via His760 loop swinging and pantothenate arm translocation, coordinated with a C-terminal ATP-grasp citrate lyase-like (CL) domain (~700-1105) housing the nucleotide-binding site where Mg²⁺-ATP phosphorylation occurs through Asp938 and Lys828 stabilization of the γ-phosphate transfer in a sequential ordered Bi Bi mechanism, breaking D2 symmetry across subunits for energetic coupling during conformational shifts from apo (open CCS) to compact intermediate states with citryl-1-phosphate and CoA at the CCS catalytic center (G281-283, S308, E599, G664-665). ACLY drives anabolic pathways by delivering acetyl-CoA for fatty acid synthase and HMGCR in nutrient-rich conditions via SREBP-1/2 activation, undergoes Akt-mediated Ser455 phosphorylation to relieve citrate allostery and boost Vmax by 3-fold while favoring ATP over CTP, couples with ACSS2 for acetate salvage during hypoxia/glucose restriction to sustain proliferation, and interfaces with epigenetics through nuclear translocation for H3K27ac at promoters of MYC/IGF2 in cancer cells, with subunit conformational barriers (His760 loops, radius of gyration drop at catalytic center formation) synchronized to biochemical steps, citrate phosphorylation, citryl-CoA formation, and cleavage, enabling ~10⁶-fold rate acceleration. Dysregulated ACLY overexpression fuels metabolic reprogramming in tumors (breast, prostate, glioblastoma) via PI3K/AKT/mTORC1 hyperactivation, promotes hepatic steatosis under high-fructose diets through ChREBP induction, and its inhibition by bempedoic acid or ND-630 reduces LDL-C and tumor growth by starving lipogenesis without impairing ketogenesis. |
| Referencias |
|---|
|
Soporte técnico
Tel: +1-832-582-8158 Ext:3
Si tiene alguna otra consulta, por favor deje un mensaje.