uitsluitend voor onderzoeksdoeleinden
Cyclopamine (11-Deoxojervine) Hedgehog Signaling Pathway Antagonist
Cat.Nr.S1146
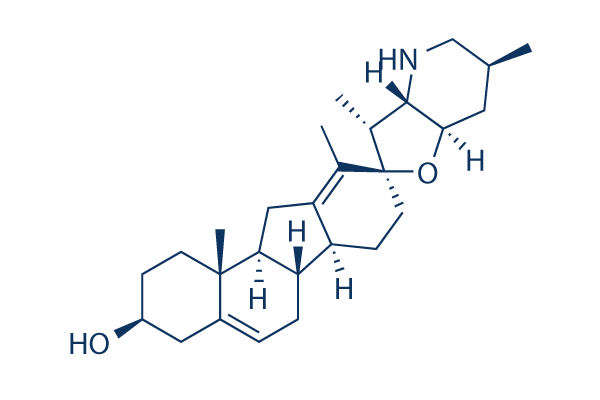
Chemische structuur
Moleculair gewicht: 411.62
Kwaliteitscontrole
| Gerelateerde doelwitten | JAK TGF-beta/Smad Wnt/beta-catenin ERK GSK-3 ROCK PKA Secretase STAT Casein Kinase |
|---|---|
| Overige Hedgehog/Smoothened Inhibitoren | SAG (Smoothened Agonist) Hydrochloride Purmorphamine GANT61 SAG (Smoothened Agonist) SANT-1 HPI-4 (Ciliobrevin A) BMS-833923 Taladegib (LY2940680) Ciliobrevin D PF-5274857 |
Celkweek, behandeling & werkconcentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| OS-RC-2 | Growth Inhibition Assay | IC50=5.8666 μM | SANGER | |||
| DOHH-2 | Growth Inhibition Assay | IC50=9.35689 μM | SANGER | |||
| no-10 | Growth Inhibition Assay | IC50=9.9039 μM | SANGER | |||
| LS-513 | Growth Inhibition Assay | IC50=11.3547 μM | SANGER | |||
| ALL-PO | Growth Inhibition Assay | IC50=11.7734 μM | SANGER | |||
| 8-MG-BA | Growth Inhibition Assay | IC50=13.1123 μM | SANGER | |||
| RPMI-8402 | Growth Inhibition Assay | IC50=15.8537 μM | SANGER | |||
| EoL-1-cell | Growth Inhibition Assay | IC50=18.5948 μM | SANGER | |||
| NALM-6 | Growth Inhibition Assay | IC50=19.0167 μM | SANGER | |||
| DEL | Growth Inhibition Assay | IC50=20.1471 μM | SANGER | |||
| SR | Growth Inhibition Assay | IC50=23.6715 μM | SANGER | |||
| 697 | Growth Inhibition Assay | IC50=26.6155 μM | SANGER | |||
| COLO-829 | Growth Inhibition Assay | IC50=26.8483 μM | SANGER | |||
| EVSA-T | Growth Inhibition Assay | IC50=27.5561 μM | SANGER | |||
| ATN-1 | Growth Inhibition Assay | IC50=31.2329 μM | SANGER | |||
| L-363 | Growth Inhibition Assay | IC50=31.7461 μM | SANGER | |||
| LAMA-84 | Growth Inhibition Assay | IC50=32.5211 μM | SANGER | |||
| NOS-1 | Growth Inhibition Assay | IC50=34.2956 μM | SANGER | |||
| BB30-HNC | Growth Inhibition Assay | IC50=34.3306 μM | SANGER | |||
| BC-1 | Growth Inhibition Assay | IC50=37.9746 μM | SANGER | |||
| IST-SL2 | Growth Inhibition Assay | IC50=38.224 μM | SANGER | |||
| D-392MG | Growth Inhibition Assay | IC50=40.2215 μM | SANGER | |||
| no-11 | Growth Inhibition Assay | IC50=40.5521 μM | SANGER | |||
| LC4-1 | Growth Inhibition Assay | IC50=40.8716 μM | SANGER | |||
| A388 | Growth Inhibition Assay | IC50=42.5848 μM | SANGER | |||
| NTERA-S-cl-D1 | Growth Inhibition Assay | IC50=42.7074 μM | SANGER | |||
| CESS | Growth Inhibition Assay | IC50=44.2232 μM | SANGER | |||
| RS4-11 | Growth Inhibition Assay | IC50=49.0938 μM | SANGER | |||
| MS-1 | Growth Inhibition Assay | IC50=50.9351 μM | SANGER | |||
| CTV-1 | Growth Inhibition Assay | IC50=51.074 μM | SANGER | |||
| D-502MG | Growth Inhibition Assay | IC50=51.6271 μM | SANGER | |||
| ML-2 | Growth Inhibition Assay | IC50=52.9195 μM | SANGER | |||
| SK-NEP-1 | Growth Inhibition Assay | IC50=53.3923 μM | SANGER | |||
| LOXIMVI | Growth Inhibition Assay | IC50=53.5884 μM | SANGER | |||
| DJM-1 | Growth Inhibition Assay | IC50=56.3391 μM | SANGER | |||
| GI-1 | Growth Inhibition Assay | IC50=56.6149 μM | SANGER | |||
| IST-MES1 | Growth Inhibition Assay | IC50=60.5493 μM | SANGER | |||
| MV-4-11 | Growth Inhibition Assay | IC50=60.6538 μM | SANGER | |||
| OVCAR-4 | Growth Inhibition Assay | IC50=63.5657 μM | SANGER | |||
| KE-37 | Growth Inhibition Assay | IC50=66.2668 μM | SANGER | |||
| D-542MG | Growth Inhibition Assay | IC50=68.4135 μM | SANGER | |||
| MHH-PREB-1 | Growth Inhibition Assay | IC50=72.8441 μM | SANGER | |||
| MRK-nu-1 | Growth Inhibition Assay | IC50=73.4705 μM | SANGER | |||
| D-247MG | Growth Inhibition Assay | IC50=73.5442 μM | SANGER | |||
| OCI-AML2 | Growth Inhibition Assay | IC50=76.9369 μM | SANGER | |||
| LP-1 | Growth Inhibition Assay | IC50=82.8731 μM | SANGER | |||
| HCC1599 | Growth Inhibition Assay | IC50=84.2837 μM | SANGER | |||
| KARPAS-45 | Growth Inhibition Assay | IC50=84.6992 μM | SANGER | |||
| BE-13 | Growth Inhibition Assay | IC50=99.0477 μM | SANGER | |||
| GCIY | Growth Inhibition Assay | IC50=99.0954 μM | SANGER | |||
| BV-173 | Growth Inhibition Assay | IC50=100.325 μM | SANGER | |||
| LB2518-MEL | Growth Inhibition Assay | IC50=100.789 μM | SANGER | |||
| KS-1 | Growth Inhibition Assay | IC50=101.639 μM | SANGER | |||
| MOLT-16 | Growth Inhibition Assay | IC50=104.986 μM | SANGER | |||
| NCI-H1770 | Growth Inhibition Assay | IC50=108.784 μM | SANGER | |||
| NCI-H82 | Growth Inhibition Assay | IC50=110.976 μM | SANGER | |||
| NCCIT | Growth Inhibition Assay | IC50=112.529 μM | SANGER | |||
| KALS-1 | Growth Inhibition Assay | IC50=115.941 μM | SANGER | |||
| LB2241-RCC | Growth Inhibition Assay | IC50=116.679 μM | SANGER | |||
| HH | Growth Inhibition Assay | IC50=117.395 μM | SANGER | |||
| HD-MY-Z | Growth Inhibition Assay | IC50=118.488 μM | SANGER | |||
| EB-3 | Growth Inhibition Assay | IC50=123.094 μM | SANGER | |||
| BL-70 | Growth Inhibition Assay | IC50=123.127 μM | SANGER | |||
| K-562 | Growth Inhibition Assay | IC50=126.245 μM | SANGER | |||
| HT-144 | Growth Inhibition Assay | IC50=133.164 μM | SANGER | |||
| PF-382 | Growth Inhibition Assay | IC50=134.361 μM | SANGER | |||
| RPMI-8226 | Growth Inhibition Assay | IC50=135.045 μM | SANGER | |||
| NCI-H1355 | Growth Inhibition Assay | IC50=135.587 μM | SANGER | |||
| LXF-289 | Growth Inhibition Assay | IC50=139.781 μM | SANGER | |||
| NCI-H69 | Growth Inhibition Assay | IC50=142.932 μM | SANGER | |||
| SK-MEL-1 | Growth Inhibition Assay | IC50=147.13 μM | SANGER | |||
| KARPAS-299 | Growth Inhibition Assay | IC50=149.12 μM | SANGER | |||
| GB-1 | Growth Inhibition Assay | IC50=149.322 μM | SANGER | |||
| CMK | Growth Inhibition Assay | IC50=149.515 μM | SANGER | |||
| MPP-89 | Growth Inhibition Assay | IC50=156.035 μM | SANGER | |||
| KU812 | Growth Inhibition Assay | IC50=161.902 μM | SANGER | |||
| REH | Growth Inhibition Assay | IC50=162.125 μM | SANGER | |||
| NEC8 | Growth Inhibition Assay | IC50=165.026 μM | SANGER | |||
| KP-N-YS | Growth Inhibition Assay | IC50=168.395 μM | SANGER | |||
| Ramos-2G6-4C10 | Growth Inhibition Assay | IC50=169.915 μM | SANGER | |||
| Becker | Growth Inhibition Assay | IC50=174.18 μM | SANGER | |||
| LB647-SCLC | Growth Inhibition Assay | IC50=175.845 μM | SANGER | |||
| LU-139 | Growth Inhibition Assay | IC50=178.019 μM | SANGER | |||
| QIMR-WIL | Growth Inhibition Assay | IC50=179.646 μM | SANGER | |||
| NCI-H1395 | Growth Inhibition Assay | IC50=179.996 μM | SANGER | |||
| NOMO-1 | Growth Inhibition Assay | IC50=182.85 μM | SANGER | |||
| GI-ME-N | Growth Inhibition Assay | IC50=187.969 μM | SANGER | |||
| KMS-12-PE | Growth Inhibition Assay | IC50=189.273 μM | SANGER | |||
| Daudi | Growth Inhibition Assay | IC50=191.128 μM | SANGER | |||
| LB996-RCC | Growth Inhibition Assay | IC50=191.699 μM | SANGER | |||
| NCI-H2107 | Growth Inhibition Assay | IC50=193.739 μM | SANGER | |||
| SK-PN-DW | Growth Inhibition Assay | IC50=194.719 μM | SANGER | |||
| MC-CAR | Growth Inhibition Assay | IC50=202.253 μM | SANGER | |||
| SNB75 | Growth Inhibition Assay | IC50=221.94 μM | SANGER | |||
| ES4 | Growth Inhibition Assay | IC50=223.783 μM | SANGER | |||
| KARPAS-422 | Growth Inhibition Assay | IC50=228.352 μM | SANGER | |||
| NCI-H1648 | Growth Inhibition Assay | IC50=229.489 μM | SANGER | |||
| ES6 | Growth Inhibition Assay | IC50=239.43 μM | SANGER | |||
| KNS-81-FD | Growth Inhibition Assay | IC50=241.197 μM | SANGER | |||
| JAR | Growth Inhibition Assay | IC50=256.225 μM | SANGER | |||
| NB1 | Growth Inhibition Assay | IC50=260.516 μM | SANGER | |||
| D-336MG | Growth Inhibition Assay | IC50=260.698 μM | SANGER | |||
| BC-3 | Growth Inhibition Assay | IC50=265.178 μM | SANGER | |||
| HCC2218 | Growth Inhibition Assay | IC50=266.415 μM | SANGER | |||
| TE-9 | Growth Inhibition Assay | IC50=266.627 μM | SANGER | |||
| LB1047-RCC | Growth Inhibition Assay | IC50=266.753 μM | SANGER | |||
| CTB-1 | Growth Inhibition Assay | IC50=269.973 μM | SANGER | |||
| NB7 | Growth Inhibition Assay | IC50=271 μM | SANGER | |||
| ST486 | Growth Inhibition Assay | IC50=277.412 μM | SANGER | |||
| HCC1187 | Growth Inhibition Assay | IC50=282.811 μM | SANGER | |||
| NCI-SNU-16 | Growth Inhibition Assay | IC50=284.248 μM | SANGER | |||
| COR-L279 | Growth Inhibition Assay | IC50=291.584 μM | SANGER | |||
| ES8 | Growth Inhibition Assay | IC50=294.182 μM | SANGER | |||
| U-698-M | Growth Inhibition Assay | IC50=298.243 μM | SANGER | |||
| HEL | Growth Inhibition Assay | IC50=309.149 μM | SANGER | |||
| KINGS-1 | Growth Inhibition Assay | IC50=310.674 μM | SANGER | |||
| KY821 | Growth Inhibition Assay | IC50=336.595 μM | SANGER | |||
| MZ1-PC | Growth Inhibition Assay | IC50=345.618 μM | SANGER | |||
| LS-411N | Growth Inhibition Assay | IC50=354.66 μM | SANGER | |||
| SIG-M5 | Growth Inhibition Assay | IC50=359.782 μM | SANGER | |||
| HT | Growth Inhibition Assay | IC50=367.711 μM | SANGER | |||
| HC-1 | Growth Inhibition Assay | IC50=367.787 μM | SANGER | |||
| NCI-H1694 | Growth Inhibition Assay | IC50=372.934 μM | SANGER | |||
| BB65-RCC | Growth Inhibition Assay | IC50=376.245 μM | SANGER | |||
| HAL-01 | Growth Inhibition Assay | IC50=379.838 μM | SANGER | |||
| ARH-77 | Growth Inhibition Assay | IC50=394.008 μM | SANGER | |||
| MZ7-mel | Growth Inhibition Assay | IC50=397.233 μM | SANGER | |||
| SIMA | Growth Inhibition Assay | IC50=403.933 μM | SANGER | |||
| DG-75 | Growth Inhibition Assay | IC50=415.698 μM | SANGER | |||
| HUTU-80 | Growth Inhibition Assay | IC50=419.185 μM | SANGER | |||
| KNS-42 | Growth Inhibition Assay | IC50=425.815 μM | SANGER | |||
| SH-4 | Growth Inhibition Assay | IC50=427.565 μM | SANGER | |||
| L-540 | Growth Inhibition Assay | IC50=431.031 μM | SANGER | |||
| NB10 | Growth Inhibition Assay | IC50=441.234 μM | SANGER | |||
| ES1 | Growth Inhibition Assay | IC50=452.753 μM | SANGER | |||
| KMOE-2 | Growth Inhibition Assay | IC50=456.711 μM | SANGER | |||
| MC116 | Growth Inhibition Assay | IC50=458.116 μM | SANGER | |||
| RCC10RGB | Growth Inhibition Assay | IC50=460.005 μM | SANGER | |||
| RL95-2 | Growth Inhibition Assay | IC50=460.237 μM | SANGER | |||
| Raji | Growth Inhibition Assay | IC50=468.143 μM | SANGER | |||
| CAS-1 | Growth Inhibition Assay | IC50=472.073 μM | SANGER | |||
| Calu-6 | Growth Inhibition Assay | IC50=475.265 μM | SANGER | |||
| KG-1 | Growth Inhibition Assay | IC50=478.44 μM | SANGER | |||
| LB771-HNC | Growth Inhibition Assay | IC50=482.232 μM | SANGER | |||
| ACN | Growth Inhibition Assay | IC50=493.599 μM | SANGER | |||
| KM12 | Growth Inhibition Assay | IC50=496.589 μM | SANGER | |||
| U2OS | Function assay | Binding affinity to wild type Smo expressed in U2OS cells by scintillation counting, Kd = 0.0124 μM. | 23063522 | |||
| U2OS | Function assay | 2 hrs | Displacement of [3H]cyclopamine from wild type Smo expressed in U2OS cells after 2 hrs by scintillation counting, Ki = 0.0127 μM. | 23063522 | ||
| TM3 | Function assay | 48 hrs | Inhibition of Hedgehog signaling pathway in mouse TM3 cells bearing pTA-8xGli-Luc reporter construct assessed as transcriptional modulation of Gli after 48 hrs by luciferase assay, IC50 = 0.046 μM. | 19091559 | ||
| HCC827 | Function assay | Displacement of [3H]-cyclopamine from SMO V404M mutant in gefitinib resistant human HCC827 cells by scintillation counting, Ki = 0.051 μM. | 28787156 | |||
| HEK293 | Function assay | 2 hrs | Displacement of BODIPY-labelled cyclopamine from human Smo receptor expressed in HEK293 cells after 2 hrs by fluorescence microscopy, IC50 = 0.064 μM. | 22268551 | ||
| Shh Light2 | Function assay | 30 hrs | Inhibition of hedgehog signaling pathway in mouse Shh Light2 cells assessed as inhibition of sonic hedgehog-induced GLI1-mediated transcriptional activity measured after 30 hrs by dual luciferase reporter gene assay, IC50 = 0.0741 μM. | 27567371 | ||
| U2OS | Function assay | Binding affinity to Smo D473H mutant expressed in U2OS cells by scintillation counting, Kd = 0.116 μM. | 23063522 | |||
| DaOY | Function assay | 48 hrs | Inhibition of Hedgehog signaling in human DaOY cells assessed as downregulation of Gli1 mRNA expression after 48 hrs by RT-PCR analysis, IC50 = 0.16 μM. | 24900716 | ||
| U2OS | Function assay | 2 hrs | Displacement of [3H]cyclopamine from Smo D473H mutant expressed in U2OS cells after 2 hrs by scintillation counting, Ki = 0.232 μM. | 23063522 | ||
| CHO | Function assay | Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay, IC50 = 0.28 μM. | 19091559 | |||
| Shh Light2 | Function assay | 40 hrs | Inhibition of SHH pathway in mouse Shh Light2 cells after 40 hrs by Gli-dependent luciferase reporter gene assay, IC50 = 0.3 μM. | 21592788 | ||
| Shh-light2 | Function assay | Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay, IC50 = 0.3 μM. | 22268551 | |||
| M210B4 | Function assay | 24 hrs | Inhibition of Hedgehog signaling in mouse M210B4 cells assessed as downregulation of Ptch mRNA expression after 24 hrs by RT-PCR analysis, IC50 = 0.43 μM. | 24900716 | ||
| Shh-Light 2 | Function assay | 2 days | Antagonist activity at Smo in mouse Shh-Light 2 cells assessed as inhibition of Shh-induced Gli1-reporter activity after 2 days by dual-luciferase reporter gene method, IC50 = 0.484 μM. | 23063522 | ||
| Shh Light2 | Function assay | Inhibition of SHH in mouse Shh Light2 cells by GLI-responsive firefly luciferase reporter gene assay, EC50 = 0.5 μM. | 19309080 | |||
| C3H10T1/2 | Function assay | Inhibition of N-terminal SHH activated pathway in mouse C3H10T1/2 cells assessed as SAG-induced cell differentiation by alkaline phosphatase assay, IC50 = 0.6 μM. | 21592788 | |||
| C3H10T1/2 | Function assay | 6 hrs | Inhibition of SAG-induced differentiation of mouse mesenchymal pluripotent C3H10T1/2 cells to alkaline phosphatase positive oeseoblasts after 6 hrs, IC50 = 0.62 μM. | 22268551 | ||
| ASZ001 | Function assay | 48 hrs | Inhibition of Hedgehog signaling in mouse ASZ001 cells assessed as downregulation of Gli1 mRNA expression after 48 hrs by RT-PCR analysis, IC50 = 0.66 μM. | 24900716 | ||
| M210B4 | Function assay | 24 hrs | Inhibition of Hedgehog signaling in mouse M210B4 cells assessed as downregulation of Gli1 mRNA expression after 24 hrs by RT-PCR analysis, IC50 = 0.8 μM. | 24900716 | ||
| medulloblastoma cells | Antiproliferative assay | Antiproliferative activity against mouse medulloblastoma cells harboring heterozygous ptch1 gene by MTT assay, EC50 = 1 μM. | 17417631 | |||
| CHO | Function assay | Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay, IC50 = 1.2 μM. | 19091559 | |||
| Shh-Light2 | Function assay | 48 hrs | Inhibition of SHH in mouse Shh-Light2 cells after 48 hrs by Gli1 reporter gene assay in presence of SAG, IC50 = 1.312 μM. | 19541490 | ||
| MEF | Function assay | Inhibition of Smo in mouse Ptch-deficient MEF cells assessed as inhibition of Shh-induced Gli1 transcriptional activity, IC50 = 1.5 μM. | 23074541 | |||
| MEF | Function assay | 30 hrs | Inhibition of Smo in mouse Ptch-deficient MEF cells assessed as inhibition of Shh-induced Gli-responsive betagalactosidase activity after 30 hrs by BetaGLo assay, IC50 = 1.9 μM. | 23074541 | ||
| neural precursor cells | Antiproliferative assay | Antiproliferative activity against mouse neural precursor cells by colony formation assay, EC50 = 13.44 μM. | 17417631 | |||
| U87 | Cytotoxicity assay | Cytotoxicity against human U87 cells assessed as viability in presence of beta glucuronidase, IC50 = 15.5 μM. | 20116904 | |||
| U87MG | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U87MG cells after 72 hrs by MTS assay, IC50 = 22.5 μM. | 22226657 | ||
| A549 | Anticancer assay | Anticancer activity against human A549 cells by MTS assay, IC50 = 49 μM. | 18221872 | |||
| DU145 | Growth inhibition assay | 5 uM | 96 hrs | Growth inhibition of human DU145 cells at 5 uM after 96 hrs | 18249125 | |
| DU145 | Growth inhibition assay | 10 uM | 96 hrs | Growth inhibition of human DU145 cells at 10 uM after 96 hrs | 18249125 | |
| HEK293 | Function assay | Inhibition of beta galactosidase in HEK293 cells | 17494766 | |||
| 22Rv | Function assay | Reduction of expression of PTCH mRNA in human 22Rv cells | 17494766 | |||
| PANC1 | Function assay | 0.2 uM | 24 hrs | Inhibition of Hh/GLI1-mediated PTCH mRNA expression in human PANC1 cells at 0.2 uM after 24 hrs by RT-PCR | 20450170 | |
| PANC1 | Function assay | 0.4 uM | 24 hrs | Inhibition of Hh/GLI1-mediated PTCH mRNA expression in human PANC1 cells at 0.4 uM after 24 hrs by RT-PCR | 20450170 | |
| Shh Light2 | Function assay | 6.25 uM | 30 hrs | Inhibition of N-palmitoylated Shh in mouse Shh Light2 cells at 6.25 uM after 30 hrs by firefly luciferase reporter gene assay | 19151731 | |
| U87MG | Function assay | 10 uM | 4 hrs | Inhibition of Hedgehog signaling pathway in human U87MG cells assessed as down regulation of Gli1 at 10 uM after 4 hrs by RT-PCR analysis | 22226657 | |
| HEK293 | Function assay | 5 uM | 10 hrs | Displacement of BODIPY-cyclopamine from human Smo expressed in HEK293 cells at 5 uM measured after 10 hrs by DAPI staining based fluorescence microscopic assay | 27736063 | |
| HEK293 | Function assay | 5 uM | 10 hrs | Displacement of BODIPY-cyclopamine from human Smo expressed in HEK293 cells at 5 uM measured after 10 hrs by FACS analysis | 27736063 | |
| C3H10T1/2 | Function assay | 55.5 to 4500 nM | Competitive inhibition of Smo in mouse C3H10T1/2 cells assessed as inhibition of SAG-induced Gli1 transcriptional activity at 55.5 to 4500 nM by qPCR analysis | 23074541 | ||
| U2OS | Function assay | 5 uM | 6 hrs | Antagonist activity at chimeric Smo 633 mutant expressed in U2OS cells coexpressing beta arrestin2-GFP assessed as inhibition of intracellular beta arrestin2-GFP aggregate formation at 5 uM after 6 hrs by confocal microscopy | 23063522 | |
| Klik om meer experimentele gegevens over de cellijn te bekijken | ||||||
Chemische informatie, Opslag en Stabiliteit
| Moleculair gewicht | 411.62 | Formule | C27H41NO2 |
Opslag (Vanaf de ontvangstdatum) | |
|---|---|---|---|---|---|
| CAS-nr. | 4449-51-8 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | 11-deoxojervine | Smiles | CC1CC2C(C(C3(O2)CCC4C5CC=C6CC(CCC6(C5CC4=C3C)C)O)C)NC1 | ||
Oplosbaarheid
|
In vitro |
Ethanol : 28 mg/mL
DMSO
: Insoluble
Water : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo Formuleringscalculator (Heldere oplossing)
Stap 1: Voer de onderstaande informatie in (Aanbevolen: Een extra dier voor het geval van verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in het gedeelte Oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-mastervloeistof: mg geneesmiddel vooraf opgelost in μL DMSO ( Concentratie mastervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de partij geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toeμL PEG300, mengen en helder maken, voeg vervolgens toeμL Tween 80, mengen en helder maken, voeg vervolgens toe μL ddH2O, mengen en helder maken.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toe μL Maïsolie, mengen en helder maken.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysische methoden zoals vortexen, echografie of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
Smoothened
(TM3Hh12 cells) 46 nM
|
|---|---|
| In vitro |
Cyclopamine remt de Hedgehog-signaleringsroute met een IC50 van 46 nM en blokkeert de activiteit van de menselijke Smo-receptor die tot expressie komt in CHO-K1-cellen in een [3H]Hh-Ag-bindingsanalyse met een IC50 van 280 nM. Deze verbinding remt de activiteit van de Hedgehog-route significant dosisafhankelijk in uit de darm afkomstige tumorcellijnen die Patched (PTCH)-mRNA tot expressie brengen, en induceert een groeiremming van die tumorcellijnen met 75-95% bij een concentratie van 3 μM, maar is ineffectief tegen colonkankercellen zonder PTCH-mRNA-expressie, wat suggereert dat de effecten van deze chemische behandeling Hedgehog-routegerelateerd zijn in plaats van algemeen cytotoxisch. Door de Hedgehog-signalering te blokkeren via directe interactie met Smo, remt het (10 μM) de proliferatie van SMOhigh cyclopamine-responsieve cellijnen L3.6sl en Panc 05.04 met 75-80% en verhoogt het de apoptose met 2,5- tot 3,5-voudig, zonder de BxPC3-SMOlow-cellijn te beïnvloeden. Deze behandeling vermindert significant het Snail-mRNA en verhoogt de E-cadherin-transcripten in de E3LZ10.7-cellijn. Onafhankelijk van de remming van celgroei, remt het significant het invasieve fenotype van Hedgehog-afhankelijke L3.6pl-cellen, wat een >500-voudige reductie veroorzaakt in het aantal transmigrerende cellen, maar niet dat van de Hedgehog-onafhankelijke cellijn Panc-1.
|
| Kinase Assay |
Hedgehog celtest
|
|
Deze test meet het eindstadium van de Hh-signaleringsroute, namelijk de transcriptionele modulatie van Gli, met behulp van Luciferase als uitlezing (Gli-Luc-test). Cyclopamine wordt voor de test bereid door seriële verdunning in DMSO en vervolgens toegevoegd aan lege testplaten. TM3Hh12-cellen (TM3-cellen die de Hh-responsieve reportergenconstructie pTA-8xGli-Luc bevatten) worden geresuspendeerd in F12 Ham's/DMEM (1:1) met 5% FBS en 15 mM Hepes pH 7.3, toegevoegd aan testplaten en gedurende ongeveer 30 minuten bij 37 °C in 5% CO2 geïncubeerd met deze verbinding. Vervolgens wordt 1 nM Hh-Ag 1.5 toegevoegd aan de testplaten en geïncubeerd bij 37 °C in aanwezigheid van 5% CO2. Na 48 uur wordt ofwel Bright-Glo ofwel MTS-reagens aan de testplaten toegevoegd en wordt de luminescentie of de absorptie bij 492 nm bepaald. De IC50-waarde, gedefinieerd als het buigpunt van de logistische curve, wordt bepaald door niet-lineaire regressie van de Gli-gestuurde luciferase-luminescentie of de absorptie van de MTS-test versus log10 (concentratie) van deze chemische stof met behulp van het R-statistische softwarepakket.
|
|
| In vivo |
Toediening van Cyclopamine in een dosis van 50 mg/kg/dag gedurende 22 dagen eradicateert de HUCCT1-xenografts in muizen zonder duidelijke bijwerkingen. Deze verbindingbehandeling in een dosis van 1,2 mg gedurende 7 dagen induceert significante apoptose van tumorcellen en vermindert de tumormassa met respectievelijk 50-60% in van Panc 05.04- en L3.6sl-afgeleide tumoren, maar niet in de BxPC3-SMOlow-tumoren. [3] Toediening van deze chemische stof alleen remt de tumormetastasen in xenografts van E3LZ10.7 en L3.6pl diepgaand en onderdrukt metastasen volledig in combinatie met gemcitabine.
|
Referenties |
|
Toepassingen
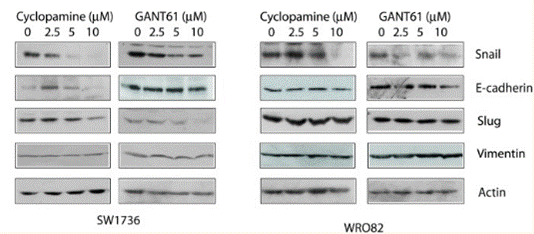
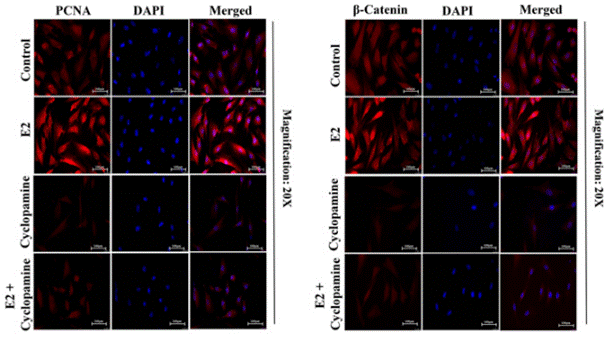
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
| Western blot | Snail / E-cadherin / Slug / Vimentin Gli1 / TGF-β1 / CXCR4 NF-κB / Cyclin D1 / MMP2 / MMP9 |
|
26859575 |
| Immunofluorescence | PCNA / β-catenin Vimentin |
|
28747625 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, kunt u een bericht achterlaten.
Veelgestelde vragen
Vraag 1:
How to reconstitute it for in vivo use in mice?
Antwoord:
One paper dissolved this compound in DMSO, and diluted it in saline: Berman DM, et al. Nature, 2003, 425(6960), 846-851. Alternatively, you can try this vehicle: 10% DMSO+30% PEG 300+5% Tween 80+ddH2O for P.O. When preparing the solution, please dissolve it in DMSO clearly first. Then add PEG300 and Tween, after they mixed well, dilute with water.
