uitsluitend voor onderzoeksdoeleinden
Triapine (3-AP) DNA/RNA Synthesis remmer
Cat.Nr.S7470
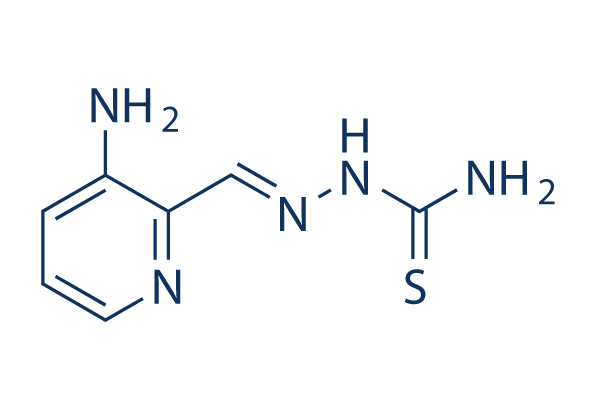
Chemische structuur
Moleculair gewicht: 195.24
Kwaliteitscontrole
| Gerelateerde doelwitten | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Overige DNA/RNA Synthesis Inhibitoren | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Carmofur YK-4-279 Halofuginone |
Celkweek, behandeling & werkconcentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| SK-N-MC | Antiproliferative assay | Antiproliferative activity against human SK-N-MC cells by MTT assay, IC50=0.31μM | 17142046 | |||
| SK-N-MC | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-MC cells after 72 hrs by MTT assay, IC50=0.26μM | 17602603 | ||
| SK-N-MC | Antiproliferative assay | Antiproliferative activity against human SK-N-MC cells by MTT assay, IC50=0.54μM | 17963372 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 18159922 | |||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-gp-negative KB-3-1 cells after 72 hrs by MTT assay, IC50=1.4μM | 19397322 | ||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-gp-negative KB-3-1 cells after 72 hrs by MTT assay, IC50=1.41254μM | 19397322 | ||
| KBV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-glycoprotein-expressing KBV1 cells after 72 hrs by MTT assay, IC50=5.88844μM | 19397322 | ||
| KBV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-glycoprotein-expressing KBV1 cells after 72 hrs by MTT assay, IC50=5.9μM | 19397322 | ||
| SK-N-MC | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-MC cells after 72 hrs by MTT assay, IC50=0.26μM | 19601577 | ||
| HCT116 | Dark cytotoxicity assay | 96 hrs | Dark cytotoxicity against human HCT116 cells expressing wild type p53 after 96 hrs by MTT assay, IC50=1.226μM | 24900837 | ||
| HCT116 | Photocytotoxicity assay | 24 hrs | Photocytotoxicity against human HCT116 cells expressing wild type p53 incubated for 24 hrs followed by light irradiation measured after 24 hrs by MTS assay in presence of ALA and FeCl3 | 24900837 | ||
| HCT116 | Function assay | 4 uM | 24 hrs | Induction of ROS generation in human HCT116 cells expressing wild type p53 at 4 uM after 24 hrs by spectrophotometry | 24900837 | |
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 27336684 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 27336684 | |||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB-3-1 cells incubated for 72 hrs by MTT assay, IC50=3.1μM | 27336684 | ||
| KBC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBC1 cells incubated for 72 hrs by MTT assay, IC50=8.9μM | 27336684 | ||
| SW480 | Function assay | 2.5 uM | 48 hrs | Induction of morphological changes in human SW480 cells assessed as induction of massive cell flattening at 2.5 uM incubated for 48 hrs by phase contrast microscopy | 27336684 | |
| HL60 | Function assay | 1 and 5 uM | 30 mins | Induction of intracellular ROS generation in human HL60 cells at 1 and 5 uM in presence of CuCl2 incubated for 30 mins by DCF-DA staining based fluorescence assay | 27336684 | |
| L1210 | Growth inhibition assay | Growth inhibition of mouse L1210 cells by MTS assay, IC50=1.3μM | 30904782 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 31614257 | |||
| WPMY-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WPMY-1 cells assessed as reduction in cell proliferation incubated for 72 hrs by MTT assay, IC50=4.96μM | 31614257 | ||
| GES-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GES-1 cells assessed as reduction in cell proliferation incubated for 72 hrs by MTT assay, IC50=5.6μM | 31614257 | ||
| HEK293 | Cytotoxicity assay | Cytotoxicity against HEK293 cells (CO-ADD:MA_007); CC50 by cell viability assay in DMEM (10% FBS) media using TC plates, by Resazurin F(560/590), CC50=2.827μM | ChEMBL | |||
| GES-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GES-1 cells after 72 hrs by MTT assay, IC50=5.4μM | ChEMBL | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50=9.68μM | ChEMBL | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50=18.85μM | ChEMBL | ||
| SMMC7721 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50=42.81μM | ChEMBL | ||
| Klik om meer experimentele gegevens over de cellijn te bekijken | ||||||
Chemische informatie, Opslag en Stabiliteit
| Moleculair gewicht | 195.24 | Formule | C7H9N5S |
Opslag (Vanaf de ontvangstdatum) | |
|---|---|---|---|---|---|
| CAS-nr. | 200933-27-3 | SDF downloaden | Opslag van stamoplossingen |
|
|
Oplosbaarheid
|
In vitro |
DMSO
: 39 mg/mL
(199.75 mM)
Water : Insoluble Ethanol : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo Formuleringscalculator (Heldere oplossing)
Stap 1: Voer de onderstaande informatie in (Aanbevolen: Een extra dier voor het geval van verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in het gedeelte Oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-mastervloeistof: mg geneesmiddel vooraf opgelost in μL DMSO ( Concentratie mastervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de partij geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toeμL PEG300, mengen en helder maken, voeg vervolgens toeμL Tween 80, mengen en helder maken, voeg vervolgens toe μL ddH2O, mengen en helder maken.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toe μL Maïsolie, mengen en helder maken.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysische methoden zoals vortexen, echografie of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
Ribonucleotide reductase
|
|---|---|
| In vitro |
Triapine (3-AP) remt potent de activiteit van ribonucleotide reductase in zowel wild-type KB als HU-resistente KB nasofaryngeale carcinoomcellen, en vertoont een breed spectrum aan antitumoractiviteit door DNA-synthese te remmen in een reeks kankercellijnen. In vitro blokkeert het ischemische neurotoxiciteit en hypoxische toxiciteit met een EC50 van respectievelijk 0,35 μM en 0,75 μM. Deze verbinding vertoont ook zijn neuroprotectieve activiteit door celdood te onderdrukken die wordt geïnduceerd door neurotoxische middelen, waaronder staurosporine, veratridine en glutamaat.
|
| Kinase Assay |
Ribonucleotide reductase-test
|
|
CDP-reductase wordt getest met behulp van Dowex 1-boraat ionenuitwisselingschromatografie. Het testmengsel bevat 0,02 μCi [14C]CDP (52,9 mCi/mmol), 3 mM dithiothreitol, 6 mM MgCl2, 30 mM HEPES, 5 mM ATP, 0,15 mM ongelabeld CDP en 10 μL cellulair extract in een eindvolume van 0,02 mL. De incubatietijd voor de reactie is 60 min, gedurende welke tijd de reactie lineair is.
|
|
| In vivo |
Bij muizen met L1210-leukemie is Triapine (3-AP) (1,25 tot 20 mg/kg) curatief voor sommige muizen zonder dodelijke toxiciteit. Deze verbinding remt ook de groei van solide tumoren in muizen M109 longcarcinoom en menselijke A2780 ovariumcarcinoom xenografts. Bovendien resulteert de combinatie met verschillende klassen van middelen die DNA beschadigen in synergetische remming van de L1210-leukemie. In een ratmodel van transiënte ischemie vermindert het het infarctvolume met 59% bij i.c.v. toediening (50 μ per rat) en met 35% bij i.v. toediening (1 mg/kg).
|
Referenties |
|
Toepassingen
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
| Western blot | p-Chk1 / Chk1 / p-CDK1/2 / CDK1/2 / p-H1 |
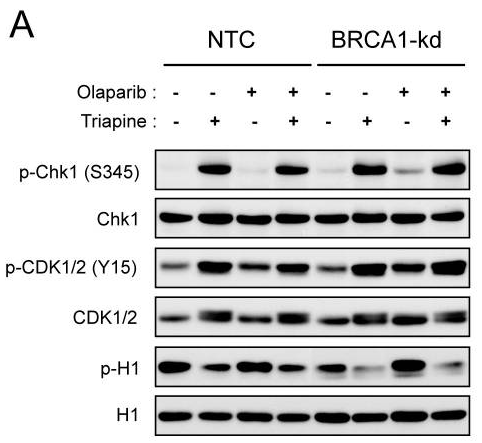
|
24413181 |
| Growth inhibition assay | Cell viability |
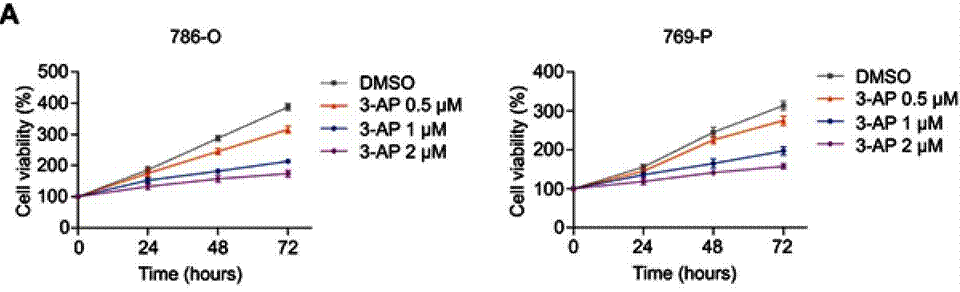
|
31118677 |
Informatie klinische proef
(gegevens van https://clinicaltrials.gov, bijgewerkt op 2024-05-22)
| NCT-nummer | Rekrutering | Aandoeningen | Sponsor/Medewerkers | Startdatum | Fasen |
|---|---|---|---|---|---|
| NCT00293345 | Completed | Anaplastic Large Cell Lymphoma|Angioimmunoblastic T-cell Lymphoma|Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue|Intraocular Lymphoma|Nodal Marginal Zone B-cell Lymphoma|Primary Central Nervous System Hodgkin Lymphoma|Primary Central Nervous System Non-Hodgkin Lymphoma|Recurrent Adult Burkitt Lymphoma|Recurrent Adult Diffuse Large Cell Lymphoma|Recurrent Adult Diffuse Mixed Cell Lymphoma|Recurrent Adult Diffuse Small Cleaved Cell Lymphoma|Recurrent Adult Hodgkin Lymphoma|Recurrent Adult Immunoblastic Large Cell Lymphoma|Recurrent Adult Lymphoblastic Lymphoma|Recurrent Adult T-cell Leukemia/Lymphoma|Recurrent Cutaneous T-cell Non-Hodgkin Lymphoma|Recurrent Grade 1 Follicular Lymphoma|Recurrent Grade 2 Follicular Lymphoma|Recurrent Grade 3 Follicular Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Marginal Zone Lymphoma|Recurrent Mycosis Fungoides/Sezary Syndrome|Recurrent Small Lymphocytic Lymphoma|Small Intestine Lymphoma|Splenic Marginal Zone Lymphoma|Stage III Adult Burkitt Lymphoma|Stage III Adult Diffuse Large Cell Lymphoma|Stage III Adult Diffuse Mixed Cell Lymphoma|Stage III Adult Diffuse Small Cleaved Cell Lymphoma|Stage III Adult Hodgkin Lymphoma|Stage III Adult Immunoblastic Large Cell Lymphoma|Stage III Adult Lymphoblastic Lymphoma|Stage III Adult T-cell Leukemia/Lymphoma|Stage III Cutaneous T-cell Non-Hodgkin Lymphoma|Stage III Grade 1 Follicular Lymphoma|Stage III Grade 2 Follicular Lymphoma|Stage III Grade 3 Follicular Lymphoma|Stage III Mantle Cell Lymphoma|Stage III Marginal Zone Lymphoma|Stage III Mycosis Fungoides/Sezary Syndrome|Stage III Small Lymphocytic Lymphoma|Stage IV Adult Burkitt Lymphoma|Stage IV Adult Diffuse Large Cell Lymphoma|Stage IV Adult Diffuse Mixed Cell Lymphoma|Stage IV Adult Diffuse Small Cleaved Cell Lymphoma|Stage IV Adult Hodgkin Lymphoma|Stage IV Adult Immunoblastic Large Cell Lymphoma|Stage IV Adult Lymphoblastic Lymphoma|Stage IV Adult T-cell Leukemia/Lymphoma|Stage IV Cutaneous T-cell Non-Hodgkin Lymphoma|Stage IV Grade 1 Follicular Lymphoma|Stage IV Grade 2 Follicular Lymphoma|Stage IV Grade 3 Follicular Lymphoma|Stage IV Mantle Cell Lymphoma|Stage IV Marginal Zone Lymphoma|Stage IV Mycosis Fungoides/Sezary Syndrome|Stage IV Small Lymphocytic Lymphoma|Unspecified Adult Solid Tumor Protocol Specific|Waldenström Macroglobulinemia |
National Cancer Institute (NCI) |
June 2006 | Phase 1 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, kunt u een bericht achterlaten.
