PDK1 (Phosphoinositide-dependent kinase-1) also known as PDPK1 (3-phosphoinositide dependent protein kinase-1) is a serine/threonine protein kinase, which functions as a master kinase to phosphorylate and activate a subgroup of the AGC family kinases such as PKB/Akt, PKC, S6K, SGK, and RSK, thus, playing a pivotal role in the PI3K/Akt signaling pathway. [show the full text]
PDK remmers (PDK Inhibitors)
| Cat.nr. | Productnaam | Informatie | Productgebruik Citaten | Productvalidaties |
|---|---|---|---|---|
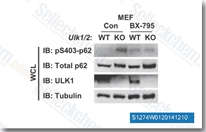
| S1274 | BX-795 | BX-795 is een krachtige en specifieke PDK1-remmer met een IC50 van 6 nM, 140- en 1600-voudig selectiever voor PDK1 dan respectievelijk PKA en PKC in celvrije assays. In vergelijking met GSK3β werd ondertussen een meer dan 100-voudige selectiviteit waargenomen voor PDK1. BX-795 moduleert autophagy via remming van ULK1. BX-795 is ook een krachtige TBK1-remmer die zowel TBK1 als IKKε blokkeert met IC50-waarden van respectievelijk 6 nM en 41 nM. |
|
|
| S0983 | JX06 | JX06 is een selectieve covalente remmer van PDK1 in cellen. Deze verbinding remt dosisafhankelijk PDK1, PDK2 en PDK3 met een IC50 van respectievelijk 0,049 μM, 0,101 μM en 0,313 μM. |
|
|
| S8615 | Sodium Dichloroacetate (DCA) | DCA (Sodium Dichloroacetate), een specifieke remmer van pyruvate dehydrogenase kinase (PDK) met IC50-waarden van respectievelijk 183 en 80 μM voor PDK2 en PDK4, heeft aangetoond de Na+-K+-2Cl- cotransporter en een mitochondriale kaliumionkanaal-as te dereprimeren. Sodium Dichloroacetate verhoogt de aanmaak van reactive oxygen species (ROS), activeert apoptose in kankercellen, en remt tumorgroei. |
|
|
| S2949 | KPLH1130 | KPLH1130 is een specifieke remmer van pyruvate dehydrogenase kinase (PDK) die macrofaagpolarisatie blokkeert en pro-inflammatoire reacties vermindert. |
PDK1 protein is composed of two well-characterized functional domains: (1) the N-terminal serine/threonine kinase domain of the AGC family, and (2) the C-terminal Pleckstrin homology (PH) domain that interacts with high affinity with both PtdIns(3,4,5)P3 and PtdIns(3,4)P2 as well as other phosphoinositides such as PtdIns(4,5)P2. PDK1 is ubiquitously expressed and constitutively active in mammalian cells. In addition to being able to constitutively phosphorylate substrates, PDK1 also works in an inducible manner in response to specific stimuli. Without stimuli, the catalytic activity of PDK1 is kept under control by limiting its access to targets, whereas upon stimulation, the different PDK1 substrates are converted into forms that can be recognized, phosphorylated and activated by PDK1. PDK1 regulates as much as 23 growth-factor-stimulated AGC kinases, containing protein kinase B (PKB/Akt) isoforms, p70 ribosomal protein S6 kinase (S6K) isoforms, p90 ribosomal protein S6 kinases (RSK), serum- and glucocorticoid-induced protein kinase (SGK) isoforms, and several protein kinase C (PKC) isoforms, by phosphorylating at the serine/threonine residues in their T-loop. [1]
The autophosphorylation of PDK1 at Ser241 in the T-loop or activation loop contributes to the catalytic activity of PDK1 upon substrate binding, and several other phosphorylation sites have also been proposed to contribute to the regulation of PDK1 activity. Upon growth factor stimulation and PtdIns(3,4,5)P3 production, both PKB/Akt and PDK1 translocate to the plasma membrane via the specific interaction of their PH domains with newly generated PtdIns(3,4,5)P3, where PDK1 could then readily phosphorylate and activate PKB/Akt, allowing PKB/Akt to phosphorylate downstream targets such as Foxo1. The mTORC1 or mTORC2 mediated phosphorylation of the hydrophobic motif of AGC kinases is essential for PDK1-mediated activation. This is achieved by creating a docking site for the binding of PDK1 to facilitate the phosphorylation of the kinases at its T-loop by PDK1. Moreover, the interaction of the hydrophobic motif binding pocket (PIF pocket) of PDK1 with the phosphorylated hydrophobic motif in the substrate induces the allosteric activation of PDK1. [1]
Therefore, through targeting a particular set of these AGC kinases, PDK1 play a critical role in regulating a wide variety of physiological processes including cell growth, proliferation, survival, development, and metabolism. PDK1 knockout mice die during early embryonic development, and the PDK1 dominant negative mutants knock-in mice are also shown to be embryonically lethal. A series of tissue-specific conditional knockout mice lacking PDK1 also confirm both the implication of PDK1 in mediating metabolic responses to insulin as well as in promoting cell viability, which proposes that selective activation of PDK1 might be a good strategy for the treatment of diabetes. However, PDK1 overexpression and amplification of the PDK1 gene are common occurrences in breast cancer and acute myeloid leukaemia. PDK1 also displays an epistatic relationship with the lipid phosphatase PTEN in the migration and malignant transformation of lymphocytes and in regulating nervous system development. Moreover, PTEN heterozygous mice expressing reduced levels of PDK1 are markedly protected from developing a wide range of tumors, indicating that PDK1 is a key effector in mediating tumorigenesis resulting from loss of PTEN and further validating PDK1 as a valuable anticancer target for the development of specific inhibitors. [1]
