Datos técnicos
| Fórmula | C31H34F2N6O2 |
||||||
| Peso molecular | 560.64 | Número CAS | 1108743-60-7 | ||||
| Solubilidad (25°C)* | In vitro | DMSO | 100 mg/mL (178.36 mM) | ||||
| Ethanol | 75 mg/mL (133.77 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Agregue los solventes al producto individualmente y en orden.) |
|
||||||
|
* <1 mg/ml significa ligeramente soluble o insoluble. * Tenga en cuenta que Selleck prueba la solubilidad de todos los compuestos internamente, y la solubilidad real puede diferir ligeramente de los valores publicados. Esto es normal y se debe a ligeras variaciones entre lotes. * Envío a temperatura ambiente (Las pruebas de estabilidad demuestran que este producto se puede enviar sin medidas de refrigeración.) |
|||||||
Preparación de soluciones madre
Actividad biológica
| Descripción | Entrectinib es un inhibidor pan-TrkA/B/C, ROS1 y ALK biodisponible por vía oral con un IC50 que oscila entre 0,1 y 1,7 nM. Entrectinib (RXDX-101) induce autophagy. Fase 2. | |||||
|---|---|---|---|---|---|---|
| Objetivos |
|
|||||
| In vitro | Entrectinib bloquea selectivamente la proliferación de líneas celulares dependientes de ALK e inhibe potentemente la señalización dependiente de ALK. Este compuesto también inhibe fuertemente el crecimiento celular de la línea celular de CPNM NCI-H2228 que presenta el reordenamiento EML4-ALK. |
|||||
| In vivo | En ratones portadores de xenoinjertos Karpas-299 y SR-786, Entrectinib (p.o.) induce la regresión tumoral completa. En ratones transgénicos NPM-ALK, este compuesto induce la regresión completa de las masas tumorales observadas en el timo y en los ganglios linfáticos. En el modelo de xenoinjerto de NB, el cotratamiento con este compuesto mejoró la eficacia de la quimioterapia convencional. |
Protocolo (de referencia)
Referencias
|
Validación de productos por parte del cliente
-
Datos de [ , , Clin Cancer Res, 2018, 24(10):2357-2369 ]
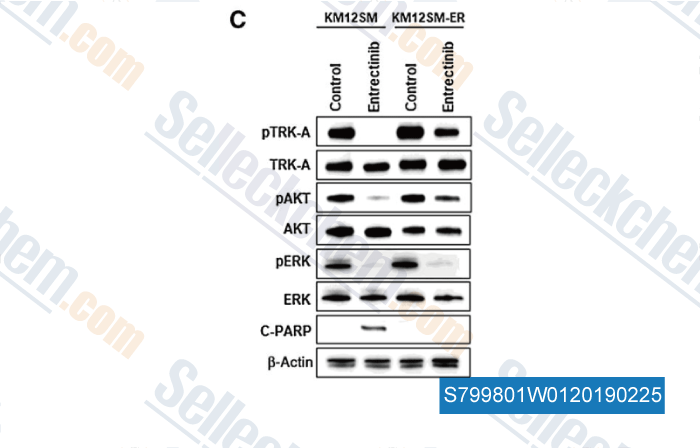
![(A) We transfected the EGFP/Eluc gene into KM12SM cells to establish KM12SM/Eluc cells. KM12SM/Eluc cells were inoculated into the brain of SCID mice. The mice were treated daily with or without entrectinib (15 mg/kg) for 37 days until the bioluminescence increased. Mean ± SE of total flux are shown in the lower panel. Then, the entrectinib-treated brain tumor was harvested at the point indicated by the orange triangle and cultured in vitro. The expanded tumor cells were named KM12SM-ER. (B) The sensitivity of KM12SM-ER and KM12SM cells to entrectinib was determined through cell viability assays, using a CCK-8 kit. The data (mean ± standard deviation [SD] of triplicate cultures) shown are representative of three independent experiments with similar results. (C) Tumor cells were treated with entrectinib (10 nmol/L) for 4 h or c-PARP for 48 h, and harvested lysates were assessed by western blotting. Data shown are representative of three independent experiments with similar results.](https://file.selleckchem.com/downloads/review/700px/Entrectinib-S799801W0120180502.gif)
-
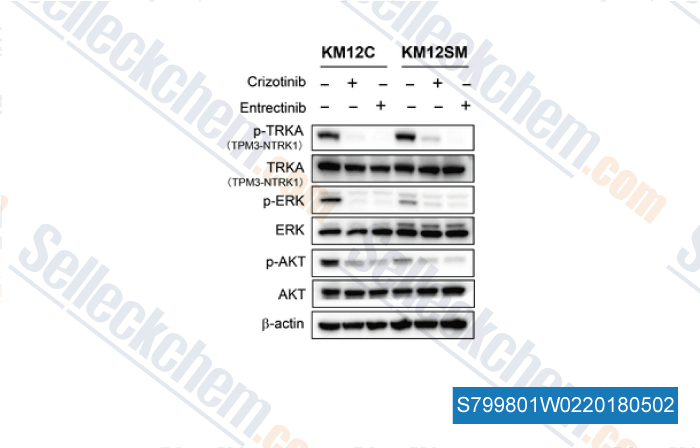
Datos de [ , , Clin Cancer Res, 2018, doi: 10.1158/1078-0432.CCR-17-1623 ]
-
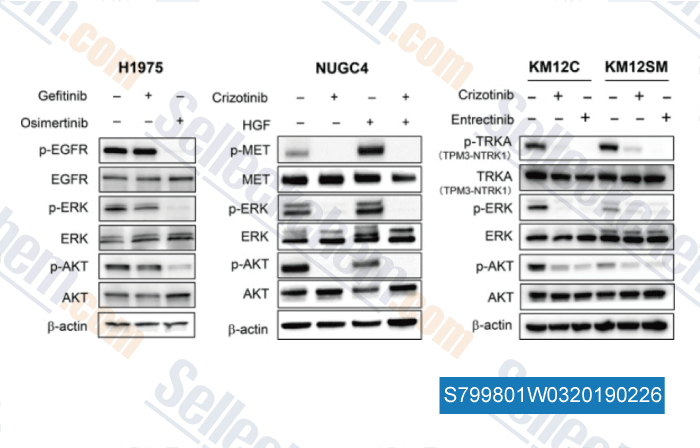
Datos de [ , , Cancer Med, 2017, 6(12):2972-2983 ]
-
Datos de [ , , Cancer Med, 2017, 6(12):2972-2983 ]
Sellecks Entrectinib (Rxdx-101) Ha sido citado por 61 Publicaciones
| Targeting proteostasis in multiple myeloma through inhibition of LTK [ Leukemia, 2025, 10.1038/s41375-025-02682-8] | PubMed: 40634511 |
| Methylstat sensitizes ovarian cancer cells to PARP-inhibition by targeting the histone demethylases JMJD1B/C [ Cancer Gene Ther, 2025, 10.1038/s41417-025-00874-z] | PubMed: 39915607 |
| Zidesamtinib Selective Targeting of Diverse ROS1 Drug-Resistant Mutations [ Mol Cancer Ther, 2025, 10.1158/1535-7163.MCT-25-0025] | PubMed: 40299789 |
| Development of PROTACs for targeted degradation of oncogenic TRK fusions [ bioRxiv, 2025, 2025.06.18.660465] | PubMed: 40666929 |
| Clinical efficacy and identification of factors confer resistance to afatinib (tyrosine kinase inhibitor) in EGFR-overexpressing esophageal squamous cell carcinoma [ Signal Transduct Target Ther, 2024, 9(1):153] | PubMed: 38937446 |
| Pharmacological inhibition of PDGF-C/neuropilin-1 interaction: A novel strategy to reduce melanoma metastatic potential [ Biomed Pharmacother, 2024, 176:116766] | PubMed: 38788599 |
| Acquired NF2 mutation confers resistance to TRK inhibition in an ex vivo LMNA::NTRK1-rearranged soft-tissue sarcoma cell model [ J Pathol, 2024, 10.1002/path.6282] | PubMed: 38613194 |
| Acquired NF2 mutation confers resistance to TRK inhibition in an ex vivo LMNA::NTRK1-rearranged soft-tissue sarcoma cell model [ J Pathol, 2024, 263(2):257-269] | PubMed: 38613194 |
| LTK mutations responsible for resistance to lorlatinib in non-small cell lung cancer harboring CLIP1-LTK fusion [ Commun Biol, 2024, 7(1):412] | PubMed: 38575808 |
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
POLÍTICA DE DEVOLUCIÓN
La Política de Devolución Incondicional de Selleck Chemical garantiza una experiencia de compra en línea fluida para nuestros clientes. Si no está satisfecho con su compra de alguna manera, puede devolver cualquier artículo(s) dentro de los 7 días posteriores a su recepción. En caso de problemas de calidad del producto, ya sean problemas relacionados con el protocolo o con el producto, puede devolver cualquier artículo(s) dentro de los 365 días a partir de la fecha de compra original. Siga las instrucciones a continuación al devolver productos.
ENVÍO Y ALMACENAMIENTO
Los productos Selleck se transportan a temperatura ambiente. Si recibe el producto a temperatura ambiente, tenga la seguridad de que el Departamento de Inspección de Calidad de Selleck ha realizado experimentos para verificar que la colocación a temperatura normal durante un mes no afectará la actividad biológica de los productos en polvo. Después de la recogida, guarde el producto de acuerdo con los requisitos descritos en la hoja de datos. La mayoría de los productos Selleck son estables en las condiciones recomendadas.
NO PARA USO HUMANO, DIAGNÓSTICO VETERINARIO O TERAPÉUTICO.
