Datos técnicos
| Fórmula | C27H20ClFN4O4 |
||||||||||
| Peso molecular | 518.92 | Número CAS | 1028486-01-2 | ||||||||
| Solubilidad (25°C)* | In vitro | DMSO | 100 mg/mL (192.7 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Agregue los solventes al producto individualmente y en orden.) |
|
||||||||||
|
* <1 mg/ml significa ligeramente soluble o insoluble. * Tenga en cuenta que Selleck prueba la solubilidad de todos los compuestos internamente, y la solubilidad real puede diferir ligeramente de los valores publicados. Esto es normal y se debe a ligeras variaciones entre lotes. * Envío a temperatura ambiente (Las pruebas de estabilidad demuestran que este producto se puede enviar sin medidas de refrigeración.) |
|||||||||||
Preparación de soluciones madre
Actividad biológica
| Descripción | Alisertib (MLN8237) es un inhibidor selectivo de Aurora A con una IC50 de 1,2 nM en un ensayo sin células, y tiene una selectividad >200 veces mayor por Aurora A que por Aurora B. Este compuesto induce la detención del ciclo celular, la apoptosis y la autophagy. Fase 3. | ||
|---|---|---|---|
| Objetivos |
|
||
| In vitro | Alisertib (MLN8237) muestra una selectividad >200 veces mayor por Aurora A que por la Aurora B estructuralmente relacionada con una IC50 de 396,5 nM, y no tiene actividad significativa contra otras 205 quinasas. El tratamiento con este compuesto (0,5 μM) inhibe la fosforilación de Aurora A en células MM1.S y OPM1, sin afectar la fosforilación de la histona H3 mediada por Aurora B. Inhibe significativamente la proliferación celular en líneas celulares de mieloma múltiple (MM) con valores de IC50 de 0,003-1,71 μM, y muestra una actividad antiproliferativa más potente contra células MM primarias y líneas celulares MM en presencia de células del estroma de la médula ósea, así como IL-6 e IGF-1, que contra células MM solas. A 0,5 μM, induce un aumento de 2 a 6 veces en la fase G2/M en células MM primarias y líneas celulares, así como apoptosis y senescencia significativas, lo que implica la regulación al alza de p53, p21 y p27, así como el clivaje de PARP, caspasa 3 y caspasa 9. Además, muestra un fuerte efecto anti-MM sinérgico con Hexadecadrol, así como un efecto aditivo con doxorrubicina y LDP-341. El tratamiento con este compuesto (0,5 μM) causa la inhibición de la formación de colonias de las líneas celulares de adenocarcinoma esofágico FLO-1, OE19 y OE33, e induce un aumento significativo en el porcentaje de células poliploides, y posteriormente un aumento en el porcentaje de células en la fase sub-G1, lo que puede mejorarse aún más en combinación con NSC 119875 (2,5 μM), lo que implica una mayor inducción de TAp73β, PUMA, NOXA, caspasa-3 clivada y PARP clivada en comparación con un tratamiento de agente único. |
||
| In vivo | Alisertib (MLN8237) reduce significativamente la carga tumoral con una inhibición del crecimiento tumoral (TGI) del 42% y 80% a 15 mg/kg y 30 mg/kg, respectivamente, y prolonga la supervivencia de los ratones en comparación con el control. |
||
| Características | Primer inhibidor de Aurora A disponible por vía oral. |
Protocolo (de referencia)
| Ensayo de quinasa: |
|
|---|---|
| Ensayo celular: |
|
| Estudio en animales: |
|
Referencias
|
Validación de productos por parte del cliente
-S113303W0120130926.gif)
-
Datos de [ Oncogene , 2014 , 33, 3550-60 ]
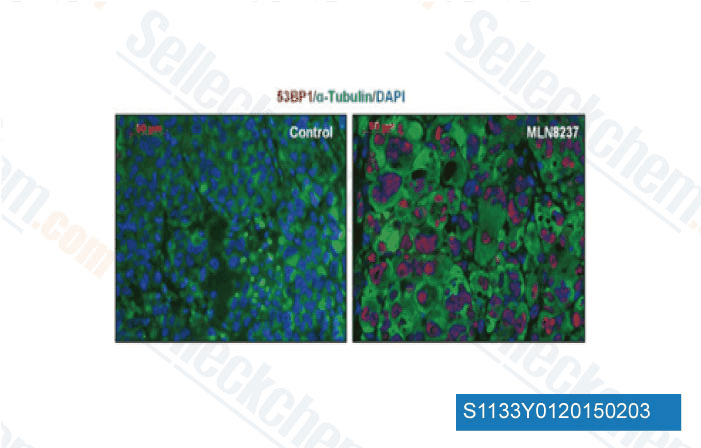
-
Datos de [ EMBO Mol Med , 2013 , 5(1), 149-66 ]
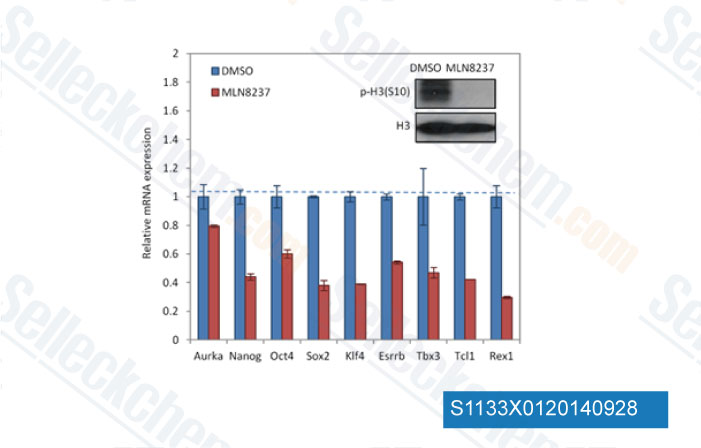
-
Datos de [ Cell Stem Cell , 2012 , 11, 179-94 ]
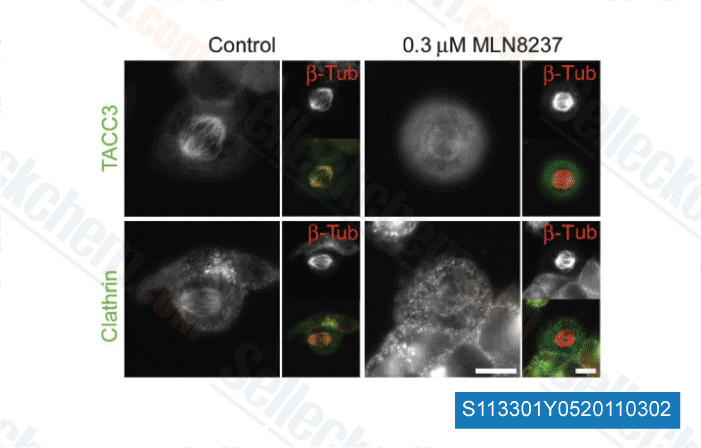
-
Datos de [ EMBO J , 2012 , 30, 906-19 ]
Sellecks Alisertib (MLN8237) Ha sido citado por 380 Publicaciones
| Centromere protection requires strict mitotic inactivation of the Bloom syndrome helicase complex [ Nat Commun, 2025, 16(1):7832] | PubMed: 40846865 |
| Aurora A regulates the material property of spindle poles to orchestrate nuclear organization at mitotic exit [ EMBO J, 2025, 10.1038/s44318-025-00564-4] | PubMed: 40940421 |
| Targeted inhibition of Aurora kinase A promotes immune checkpoint inhibition efficacy in human papillomavirus-driven cancers [ J Immunother Cancer, 2025, 13(1)e009316] | PubMed: 39773561 |
| An Aurora kinase A-BOD1L1-PP2A B56 axis promotes chromosome segregation fidelity [ Cell Rep, 2025, 44(2):115317] | PubMed: 39970043 |
| The AURKA inhibitor alters the immune microenvironment and enhances targeting B7-H3 immunotherapy in glioblastoma [ JCI Insight, 2025, e173700] | PubMed: 39928563 |
| CDK1-mediated phosphorylation of LDHA fuels mitosis through LDHB-dependent lactate oxidation [ EMBO Rep, 2025, 10.1038/s44319-025-00573-8] | PubMed: 40940446 |
| Cellular senescence as a prognostic marker for predicting breast cancer progression in 2D and 3D organoid models [ Biomed Pharmacother, 2025, 189:118324] | PubMed: 40616881 |
| Actionable heterogeneity of hepatocellular carcinoma therapy-induced senescence [ Cancer Immunol Immunother, 2025, 74(7):207] | PubMed: 40374812 |
| Aurora B maintains spherical shape of mitotic cells via simultaneously stabilizing myosin II and vimentin [ J Mol Cell Biol, 2025, mjaf023] | PubMed: 40795355 |
| O 6-methylguanine DNA methyltransferase (MGMT) expression in U1242 glioblastoma cells enhances in vitro clonogenicity, tumor implantation in vivo, and sensitivity to alisertib-carboplatin combination treatment [ Front Cell Neurosci, 2025, 19:1552015] | PubMed: 40336841 |
POLÍTICA DE DEVOLUCIÓN
La Política de Devolución Incondicional de Selleck Chemical garantiza una experiencia de compra en línea fluida para nuestros clientes. Si no está satisfecho con su compra de alguna manera, puede devolver cualquier artículo(s) dentro de los 7 días posteriores a su recepción. En caso de problemas de calidad del producto, ya sean problemas relacionados con el protocolo o con el producto, puede devolver cualquier artículo(s) dentro de los 365 días a partir de la fecha de compra original. Siga las instrucciones a continuación al devolver productos.
ENVÍO Y ALMACENAMIENTO
Los productos Selleck se transportan a temperatura ambiente. Si recibe el producto a temperatura ambiente, tenga la seguridad de que el Departamento de Inspección de Calidad de Selleck ha realizado experimentos para verificar que la colocación a temperatura normal durante un mes no afectará la actividad biológica de los productos en polvo. Después de la recogida, guarde el producto de acuerdo con los requisitos descritos en la hoja de datos. La mayoría de los productos Selleck son estables en las condiciones recomendadas.
NO PARA USO HUMANO, DIAGNÓSTICO VETERINARIO O TERAPÉUTICO.
